Question: (a) What is the difference between an ideal and non-ideal solution? (b) The activity coefficients for two components in a binary solution at 25C at
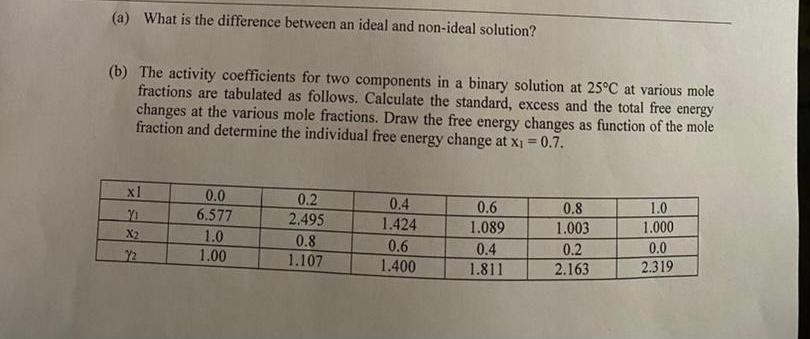
(a) What is the difference between an ideal and non-ideal solution? (b) The activity coefficients for two components in a binary solution at 25C at various mole fractions are tabulated as follows. Calculate the standard, excess and the total free energy changes at the various mole fractions. Draw the free energy changes as function of the mole fraction and determine the individual free energy change at x1=0.7. (a) What is the difference between an ideal and non-ideal solution? (b) The activity coefficients for two components in a binary solution at 25C at various mole fractions are tabulated as follows. Calculate the standard, excess and the total free energy changes at the various mole fractions. Draw the free energy changes as function of the mole fraction and determine the individual free energy change at x1=0.7
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


