Question: a) Write correct coefficients for the combustion reaction. b) Consider air to be 21.0 mole percent O2 and the balance N2. For the case of
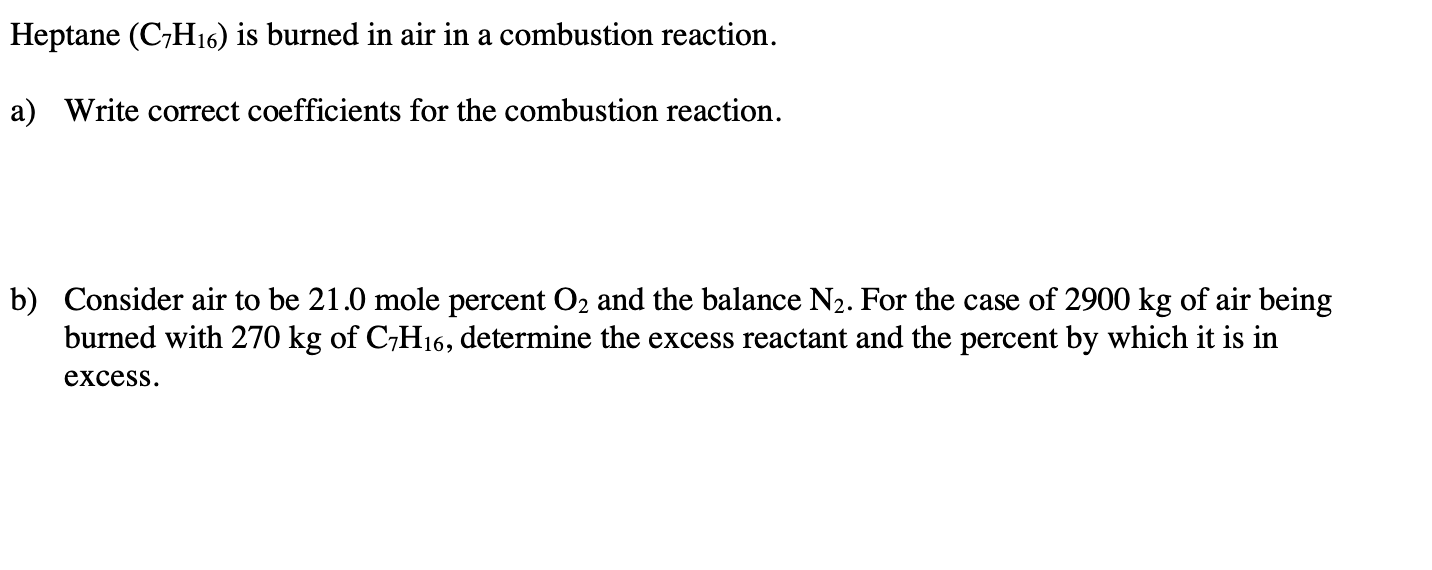
a) Write correct coefficients for the combustion reaction. b) Consider air to be 21.0 mole percent O2 and the balance N2. For the case of 2900kg of air being burned with 270kg of C7H16, determine the excess reactant and the percent by which it is in excess
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


