Question: A1-PartB: Problem 4.5 H2SO4 Set up a stoichiometric table for each of the following reactions and express the concentration of each species in the reaction
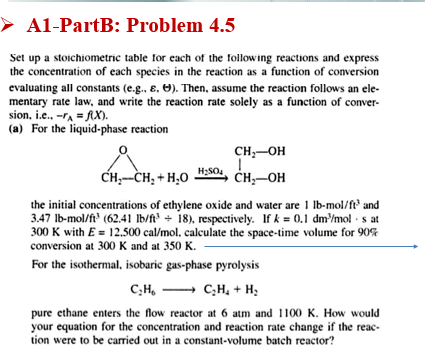
A1-PartB: Problem 4.5 H2SO4 Set up a stoichiometric table for each of the following reactions and express the concentration of each species in the reaction as a function of conversion evaluating all constants (e.g., E, O). Then, assume the reaction follows an ele- mentary rate law, and write the reaction rate solely as a function of conver- sion, i.e..-"A=fx). (a) For the liquid-phase reaction CH;-OH | CH-CH2 + H20 CH-OH the initial concentrations of ethylene oxide and water are 1 lb-mol/ft and 3.47 lb-mol/ft (62.41 lb/ft + 18), respectively. If k = 0.1 dm mols at 300 K with E = 12.500 cal/mol. calculate the space-time volume for 90% conversion at 300 K and at 350 K. For the isothermal, isobaric gas-phase pyrolysis C.H. C.H. + H2 pure ethane enters the flow reactor at 6 atm and 1100 K. How would your equation for the concentration and reaction rate change if the reac- tion were to be carried out in a constant-volume batch reactor
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


