Question: A2 - Fundamental Equilibria Consider the theoretical reaction shown below: 2A(aq)+B(aq)C(aq)+2D(aq) If Gmon for this process is 83.20kJ/mol determine the equilibrium constant for the reaction
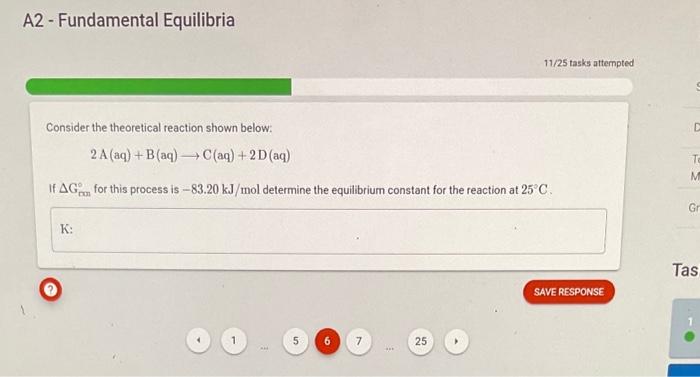
A2 - Fundamental Equilibria Consider the theoretical reaction shown below: 2A(aq)+B(aq)C(aq)+2D(aq) If Gmon for this process is 83.20kJ/mol determine the equilibrium constant for the reaction at 25C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


