Question: Accessibility Mode Sim dvance Study Assignment 1. A current of 100.mA is used to electrolyze a dilute solution of sulfuric acid for 1 hour and
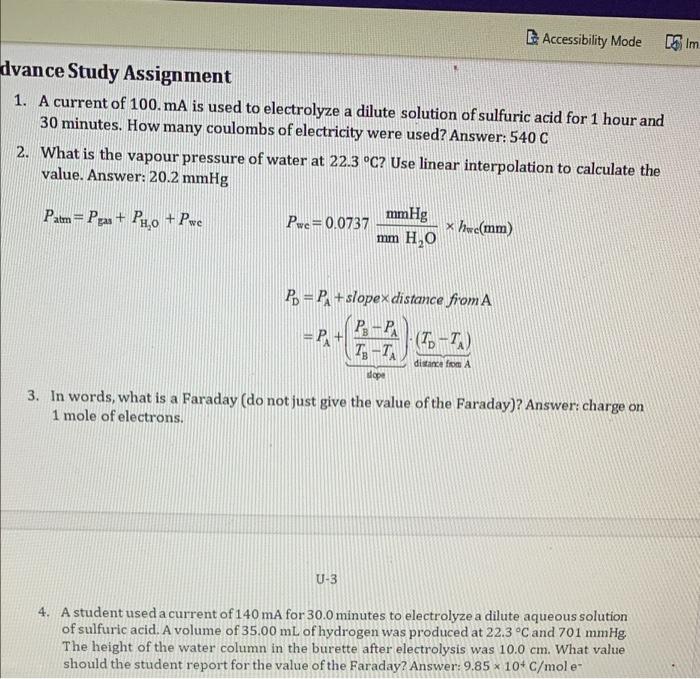
Accessibility Mode Sim dvance Study Assignment 1. A current of 100.mA is used to electrolyze a dilute solution of sulfuric acid for 1 hour and 30 minutes. How many coulombs of electricity were used? Answer: 540 C 2. What is the vapour pressure of water at 22.3 C? Use linear interpolation to calculate the value. Answer: 20.2 mmHg mmHg Patm= Pps+ P4,0 + Pwe Pwe=0.0737 x hwe(mm) mm H2O Po = P + slopex distance from A Pg - PA + (To-T) T-TA = PA distance from A dope 3. In words, what is a Faraday (do not just give the value of the Faraday)? Answer: charge on 1 mole of electrons. U-3 4. A student used a current of 140 mA for 30.0 minutes to electrolyze a dilute aqueous solution of sulfuric acid. A volume of 35.00 mL of hydrogen was produced at 22.3 C and 701 mmHg The height of the water column in the burette after electrolysis was 10.0 cm. What value should the student report for the value of the Faraday? Answer: 9.85 * 10* C/mole X
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


