Question: According to a standard reference, the Henry's law constant for methane, CH4, dissolved in water at 25C is 1.4105mol/m3Pa. What is the concentration of methane,
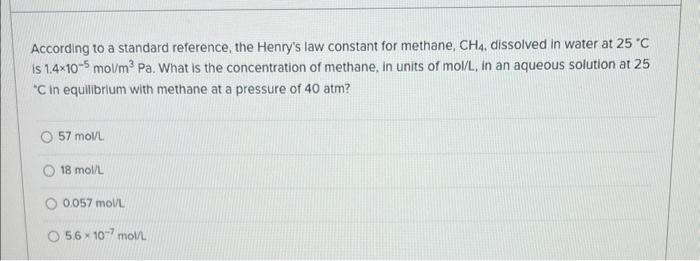
According to a standard reference, the Henry's law constant for methane, CH4, dissolved in water at 25C is 1.4105mol/m3Pa. What is the concentration of methane, in units of mol/L, in an aqueous solution at 25 C in equilibrium with methane at a pressure of 40atm ? 57moll 18molh 0.057moll 5.6107mol/L
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


