Question: Acetic acid can be produced through the given balanced reaction and with the process shown in the image. Ca(C2H3O2)2+H2SO4CaSO4+2C2H4O2 In the process, 535lb/h of fresh
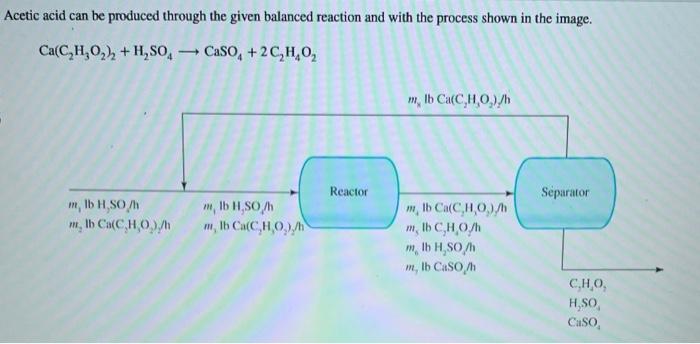
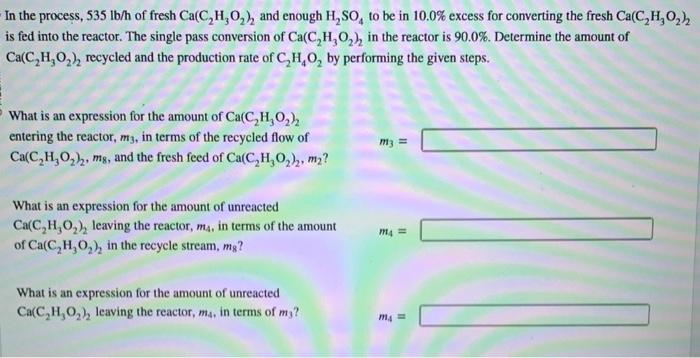
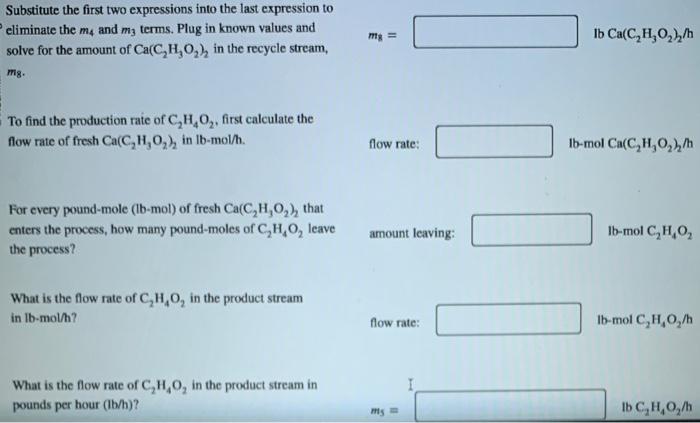
Acetic acid can be produced through the given balanced reaction and with the process shown in the image. Ca(C2H3O2)2+H2SO4CaSO4+2C2H4O2 In the process, 535lb/h of fresh Ca(C2H3O2)2 and enough H2SO4 to be in 10.0% excess for converting the fresh Ca(C2H3O2)2 is fed into the reactor. The single pass conversion of Ca(C2H3O2)2 in the reactor is 90.0%. Determine the amount of Ca(C2H3O2)2 recycled and the production rate of C2H4O2 by performing the given steps. What is an expression for the amount of Ca(C2H3O2)2 entering the reactor, m3, in terms of the recycled flow of m3= Ca(C2H3O2)2,m8, and the fresh feed of Ca(C2H3O2)2,m2 ? What is an expression for the amount of unreacted Ca(C2H3O2)2 leaving the reactor, m4, in terms of the amount m4= of Ca(C2H3O2)2 in the recycle stream, m8 ? What is an expression for the amount of unreacted Ca(C2H3O2)2 leaving the reactor, m4, in terms of m3 ? m4= Substitute the first two expressions into the last expression to eliminate the m4 and m3 terms. Plug in known values and mg= IbCa(C2H3O2)2/h solve for the amount of Ca(C2H3O2)2 in the recycle stream, m8. To find the production rate of C2H4O2, first calculate the flow rate of fresh Ca(C2H3O2)2 in Ib-mol/h. flow rate: Ib-mol Ca(C2H3O2)2/h For every pound-mole (lb-mol) of fresh Ca(C2H3O2)2 that enters the process, how many pound-moles of C2H4O2 leave amount leaving: the process? What is the flow rate of C2H4O2 in the product stream in lbmol/h ? flow rate: lbmolC2H4O2/h What is the flow rate of C2H4O2 in the product stream in pounds per hour (lb/h)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


