Question: acid base extraction need help with question 1 & 2 1. Discuss how the results of part A are used in successfully separating the three

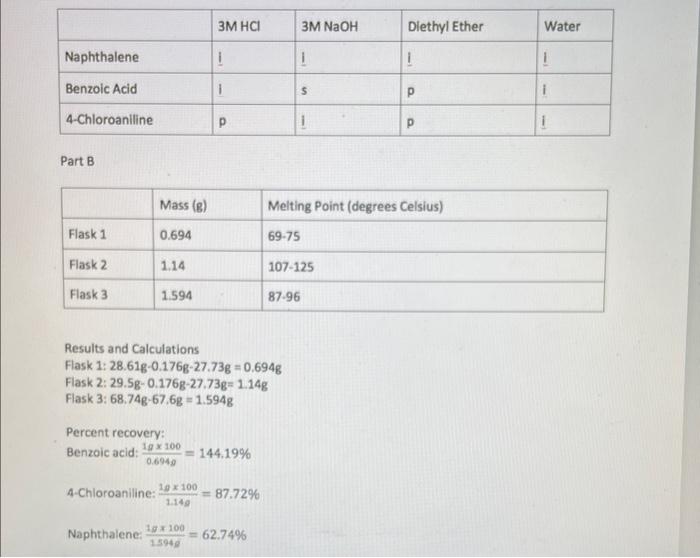
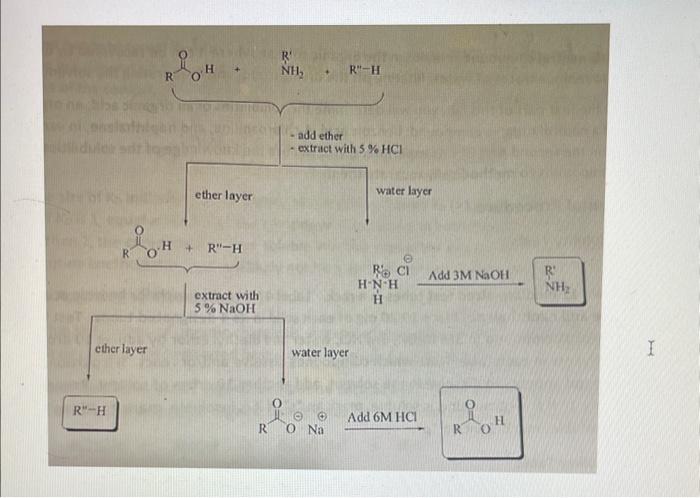
1. Discuss how the results of part A are used in successfully separating the three organic compounds in part B. 2. Calculate the composition of the unknown mixture in weight percent Part B Results and Calculations Flask 1: 28.61g-0.176g-27.73g =0.694g Flask 2: 29.5g0.176g27.73g=1.14g Flask 3: 68.74g67.6g=1.594g Percent recovery: Benzoic acid: 0.69401g100=144.19% 4.Chloroaniline: 1.14g1g100=87.72% Naphthalene: 1.594g1g100=62.74% ether layer water layer RH R 0NaAdd6MHCl=ROH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


