Question: acid-base titration lab! any help i can get would be amazing:) V25ml HCl C = 0.1 M Acid Base Titration LAB V= C2= 1st pink

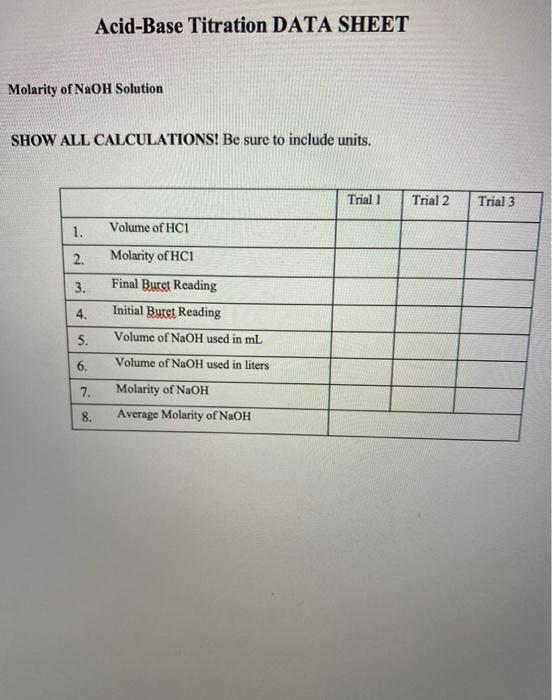




V25ml HCl C = 0.1 M Acid Base Titration LAB V= C2= 1st pink : 11 mL in burette, somLin 2nd pink: 10.SmL in burette, somLin Burette-NaOH~100mL Pipette-Ac~50mL Phenolphthalein indicatur coloruss in acid, pink in base transition point Nevtralization point
Step by Step Solution
3.50 Rating (153 Votes )
There are 3 Steps involved in it
Hope ... View full answer

Get step-by-step solutions from verified subject matter experts


