Question: Activity 1: Phase Equilibrium Diagrams - Binary System As a group, construct a Phase Equilibrium Diagram representing a binary alloy system of two elements A

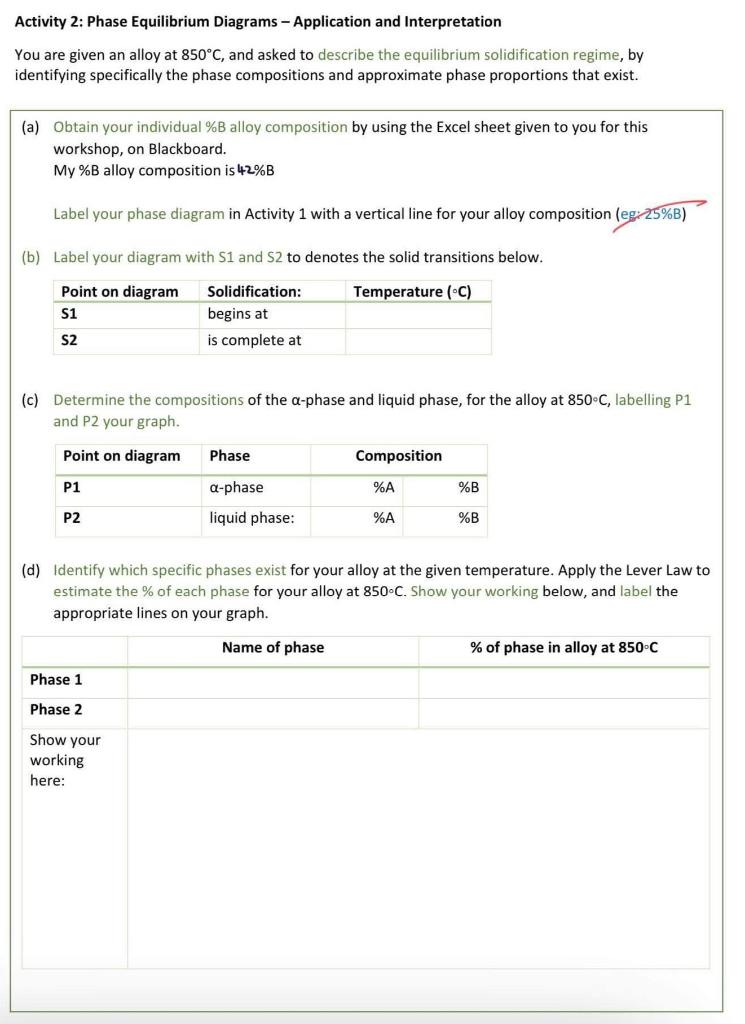

Activity 1: Phase Equilibrium Diagrams - Binary System As a group, construct a Phase Equilibrium Diagram representing a binary alloy system of two elements A and B, using the following data: (a) A melts at 1290C and B melts at 950C (b) A is soluble in B in the solid state to the extent of 12%A in B at 720C, and 4%A in B at room temperature (20C) (c) B is soluble in A in the solid state to the extent of 18%B in A at 720C and 8%B in A at room temperature (20C) (d) A eutectic is formed at 720C at a composition of 32%A Assume that the solid solution of B in A is known as a-phase, and that the solid solution of A in B is known as b - phase. Label all phase fields and phase boundary lines (solidus, liquidus, solvus). You may draw your graph here, or copy and paste it in. Assume lines joining data are linear. Activity 2: Phase Equilibrium Diagrams - Application and Interpretation You are given an alloy at 850C, and asked to describe the equilibrium solidification regime, by identifying specifically the phase compositions and approximate phase proportions that exist. (a) Obtain your individual \%B alloy composition by using the Excel sheet given to you for this workshop, on Blackboard. My \%B alloy composition is 42%B Label your phase diagram in Activity 1 with a vertical line for your alloy composition (egi 25%B ) (b) Label your diagram with S1 and S2 to denotes the solid transitions below. (c) Determine the compositions of the -phase and liquid phase, for the alloy at 850C, labelling P1 and P2 your graph. (d) Identify which specific phases exist for your alloy at the given temperature. Apply the Lever Law to estimate the % of each phase for your alloy at 850C. Show your working below, and label the appropriate lines on your graph. Activity 3: Materials Selection Exercise Below is a list of materials: Select from this list one material that you think is best suited for each of the following applications, and give at least one reason or selection criteria for each choice: (NB. Materials listed can be selected
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


