Question: All amino acids have two ionizable functional groups: an a-amino group (average pKa of 9.4) and an a-carboxylic acid group (average pK, of 2.2).
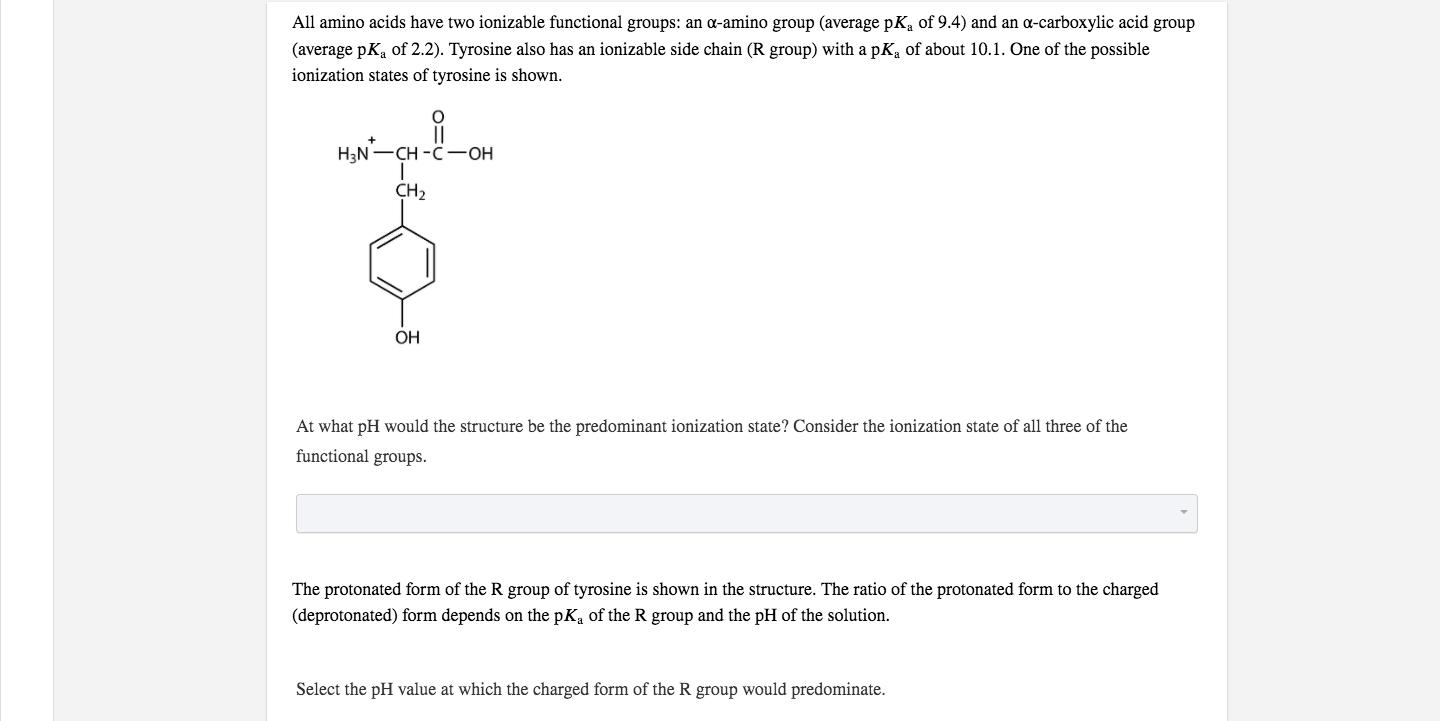
All amino acids have two ionizable functional groups: an a-amino group (average pKa of 9.4) and an a-carboxylic acid group (average pK, of 2.2). Tyrosine also has an ionizable side chain (R group) with a pK of about 10.1. One of the possible ionization states of tyrosine is shown. H3N-CH -C-OH CH2 OH At what pH would the structure be the predominant ionization state? Consider the ionization state of all three of the functional groups. The protonated form of the R group of tyrosine is shown in the structure. The ratio of the protonated form to the charged (deprotonated) form depends on the pKa of the R group and the pH of the solution. Select the pH value at which the charged form of the R group would predominate.
Step by Step Solution
3.38 Rating (148 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


