Question: all data given, calculate paper. Recorded Data Unknown Number or Letter Trial 1 Trial 2 1. Mass of dry crucible and cover (This would be

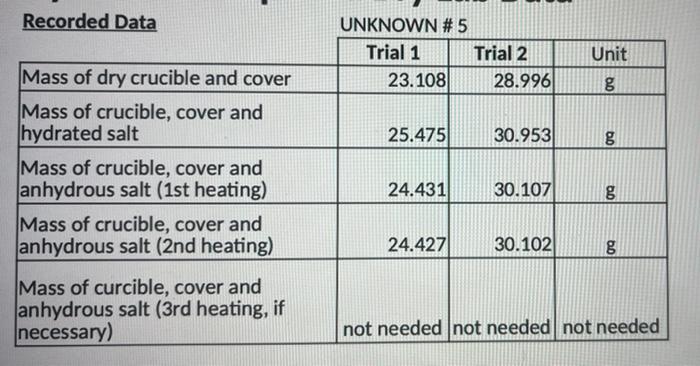





Recorded Data Unknown Number or Letter Trial 1 Trial 2 1. Mass of dry crucible and cover (This would be measured after heating the washed crucible to dryness in step 2.) 2. Mass of crucible, cover and hydrated salt 3. Mass of crucible, cover and anhydrous salt (15 heating) 4. Mass of crucible, cover and anhydrous salt (2nd heating) 5.-Mass of crucible, cover and anhydrous salt 13- heating, if necessary) Calculations Show your work for all calculations in Trial 1 in the spaces provided. Trial 1 Trial 2 1. Mass of hydrated salt 2. Mass of anhydrous salt (after last heating) 3. Mass of water lost during heating 4. Mass percent of water in the hydrated salt (experimental mass percent) I Average experimental mass percent of water Recorded Data UNKNOWN #5 Trial 1 Trial 2 23.108 28.996 Unit g 25.475 30.953 09 Mass of dry crucible and cover Mass of crucible, cover and hydrated salt Mass of crucible, cover and anhydrous salt (1st heating) Mass of crucible, cover and anhydrous salt (2nd heating) 24.431) 30.107 g 24.427) 30.102 09 Mass of curcible, cover and anhydrous salt (3rd heating, if necessary) not needed not needed not needed Known Hydrated Salts: The possible unknowns used in this lab are listed below. Determine the mass percent of water in the following hydrated salts. Show all work in the space provided. Hydrate Mass Percent of Water Barium Chloride Dihydrate, BaCl2.2H2O Calcium Chloride Dihydrate, CaCl2-2H20 Magnesium Sulfate Heptahydrate, MgSO4.7H2O Zinc Sulfate Heptahydrate, ZnSO4.7H30 Sodium Carbonate Decahydrate, Na2CO310H20 Compare your average experimental mass percent of water in your unknown hydrated sample to the mass percent of water in the known hydrated salts list above. The identity of your unknown hydrated salt is: Given: Percent Error = | experimental mass % - known mass % known mass % x 100 1. Does the experimental) mass percent of water in your unknown hydrated salt match the (known) mass percent of water in the hydrated salt you identified as your unknown? If not, determine the percent error of your average (experimental) mass percent using the equation given above. 2. Percent error is one method of evaluating the accuracy of your experimental results. If the percent error you determined in #1 above is more than 10%, give two possible sources of error that could have occurred during the experiment, and explain how that error would have impacted your results. 3. When multiple trials are done in an experiment, precision should be evaluated as well as accuracy. While accuracy evaluates how close your average result is to the known value, precision looks at how close your results from all trials are to each other. Looking at your results for trials 1 and 2 for the mass percent of water in your unknown, and looking at the percent error you determined in #1 above, do you think your experimental results revealed better accuracy or better precision? Explain your answer. Mass of Crucible and Lid 25.1010 g Mass of Crucible, Lid and Hydrate 26.1012 g 25.7728 g Mass of Crucible, Lid and Anhydrous Salt (13 Heating) Mass of Crucible, Lid and Anhydrous Salt (2nd Heating) Mass of Crucible, Lid and Anhydrous Salt (3rd Heating) 25.6730 g 25.6534 g A) What was the mass of the hydrated sample used in this experiment? B) What was the mass of the anhydrous salt after the 3rd heating? C) What was the mass of water lost during the experiment? D) What was the mass percent of water in the hydrated salt? E) The student was told that the unknown was aluminum chloride hexahydrate. Determine the mass percent of water in aluminum chloride hexahydrate. Do her experimental results match the known mass of percent of water in aluminum chloride hexahydrate? F) Looking at her data, was it necessary for her to do a 3rd heating? Why or why not? If she had not done a 3rd heating, how would that have affected her results? 4. A student obtained the following data of an aluminum chloride hydrate, AlCl3.XH20. 25.1010 g Mass of Crucible and Lid 26.1012 g 25.7728 g Mass of Crucible, Lid and Hydrate Mass of Crucible, Lid and Anhydrous Salt (1st Heating) Mass of Crucible, Lid and Anhydrous Salt (2nd Heating) Mass of Crucible, Lid and Anhydrous Salt (3rd Heating) 25.6730 g 25.6534 g
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


