Question: all please Context: Hydroxymethylbilane synthase is the enzyme responsible for heme production. Heme is an essential component of hemoproteins, such as hemoglobin. People suffering from
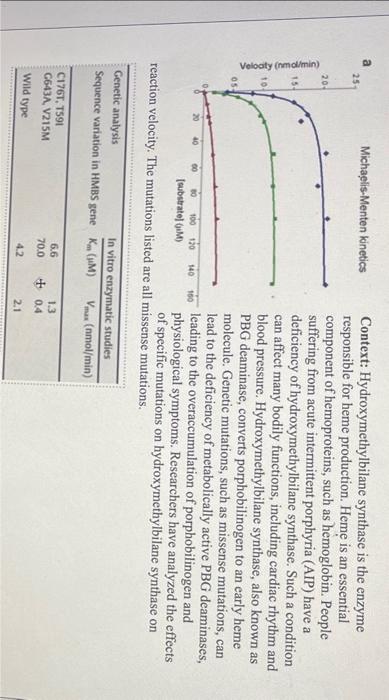
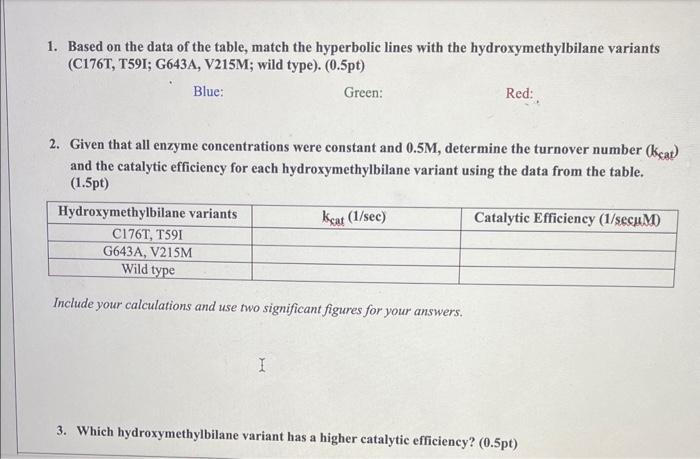

Context: Hydroxymethylbilane synthase is the enzyme responsible for heme production. Heme is an essential component of hemoproteins, such as hemoglobin. People suffering from acute intermittent porphyria (AIP) have a deficiency of hydroxymethylbilane synthase. Such a condition can affect many bodily functions, including cardiac rhythm and blood pressure. Hydroxymethylbilane synthase, also known as PBG deaminase, converts porphobilinogen to an early heme molecule. Genetic mutations, such as missense mutations, can lead to the deficiency of metabolically active PBG deaminases, leading to the overaccumulation of porphobilinogen and physiological symptoms. Researchers have analyzed the effects of specific mutations on hydroxymethylbilane synthase on nissense mutations. 1. Based on the data of the table, match the hyperbolic lines with the hydroxymethylbilane variants (C176T, T59I; G643A, V215M; wild type). (0.5pt) Blue: Green: Red: 2. Given that all enzyme concentrations were constant and 0.5M, determine the turnover number (kcat) and the catalytic efficiency for each hydroxymethylbilane variant using the data from the table. (1.5pt) Include your calculations and use two significant figures for your answers. 3. Which hydroxymethylbilane variant has a higher catalytic efficiency? (0.5pt) 3. Which hydroxymethylbilane variant has a higher catalytic efficiency? (0.5pt) 4. Assuming only mutant variants of hydroxymethylbilane result in acute intermittent porphyria (AIP), a catalytic efficiency (1/secuM) lower than what value increases the likelihood of AIP? (0.5pt) 5. What does a lower catalytic efficiency entail in terms of substrate-to-product conversion and substrate affinity? Refer to the equation of catalytic efficiency. (0.5pt) 6. Using the Michaelis-Menten equation, determine at what substrate concentration (M) the reaction rate is 80% of Vmax for the wild type variant? (1pt) Include your calculations. 7. Based on the curves observed within the graph, do any of the hydroxymethylbilane variants exhibit cooperativity? Consider the shape of the curves. ( 0.5pt) Context: Hydroxymethylbilane synthase is the enzyme responsible for heme production. Heme is an essential component of hemoproteins, such as hemoglobin. People suffering from acute intermittent porphyria (AIP) have a deficiency of hydroxymethylbilane synthase. Such a condition can affect many bodily functions, including cardiac rhythm and blood pressure. Hydroxymethylbilane synthase, also known as PBG deaminase, converts porphobilinogen to an early heme molecule. Genetic mutations, such as missense mutations, can lead to the deficiency of metabolically active PBG deaminases, leading to the overaccumulation of porphobilinogen and physiological symptoms. Researchers have analyzed the effects of specific mutations on hydroxymethylbilane synthase on nissense mutations. 1. Based on the data of the table, match the hyperbolic lines with the hydroxymethylbilane variants (C176T, T59I; G643A, V215M; wild type). (0.5pt) Blue: Green: Red: 2. Given that all enzyme concentrations were constant and 0.5M, determine the turnover number (kcat) and the catalytic efficiency for each hydroxymethylbilane variant using the data from the table. (1.5pt) Include your calculations and use two significant figures for your answers. 3. Which hydroxymethylbilane variant has a higher catalytic efficiency? (0.5pt) 3. Which hydroxymethylbilane variant has a higher catalytic efficiency? (0.5pt) 4. Assuming only mutant variants of hydroxymethylbilane result in acute intermittent porphyria (AIP), a catalytic efficiency (1/secuM) lower than what value increases the likelihood of AIP? (0.5pt) 5. What does a lower catalytic efficiency entail in terms of substrate-to-product conversion and substrate affinity? Refer to the equation of catalytic efficiency. (0.5pt) 6. Using the Michaelis-Menten equation, determine at what substrate concentration (M) the reaction rate is 80% of Vmax for the wild type variant? (1pt) Include your calculations. 7. Based on the curves observed within the graph, do any of the hydroxymethylbilane variants exhibit cooperativity? Consider the shape of the curves. ( 0.5pt)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


