Question: Electron Pair Molecular Molecule Lewis Dot Structure Arrangement Geometry Polarity H2S H-S-H CH,CI H-E- O3 O= st = SiO2 %3. Both C's: Both C's:
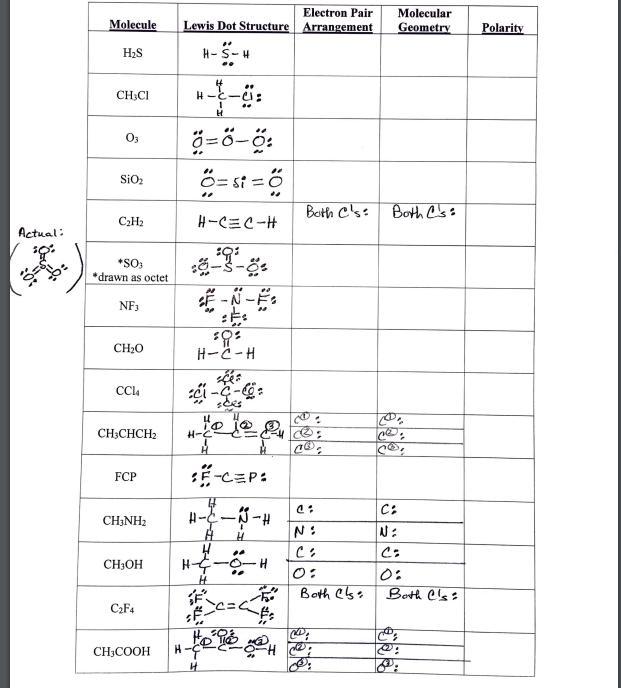
Electron Pair Molecular Molecule Lewis Dot Structure Arrangement Geometry Polarity H2S H-S-H CH,CI H-E- O3 O= st = SiO2 %3. Both C's: Both C's: C2H2 H-C=C-H Actual: *SO *drawn as octet -3-8: NF3 CH20 H-2-H CC4 CH;CHCH2 -CEP: FCP C: CH;NH2 N: N: CH3OH 0: Both es: Both Cs: C2F4 CH3COOH H
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


