Question: all the data is here jist need help with the rest amd see if my answers i filled in are correct! thank you! Standardization of
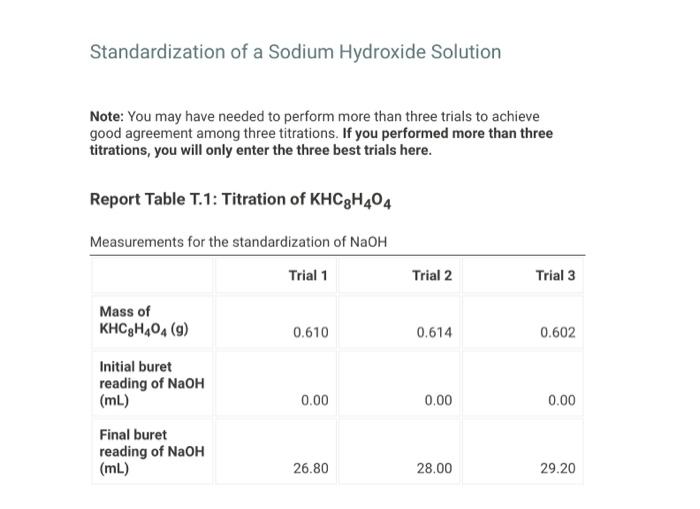

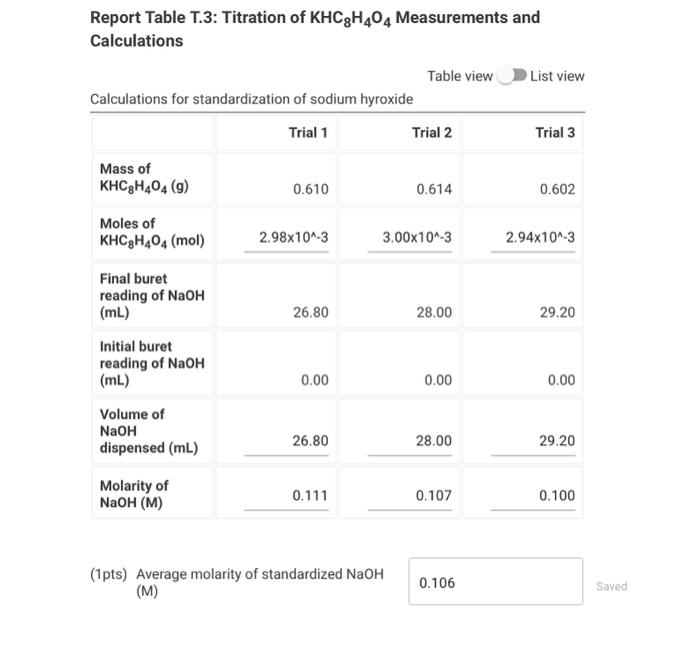
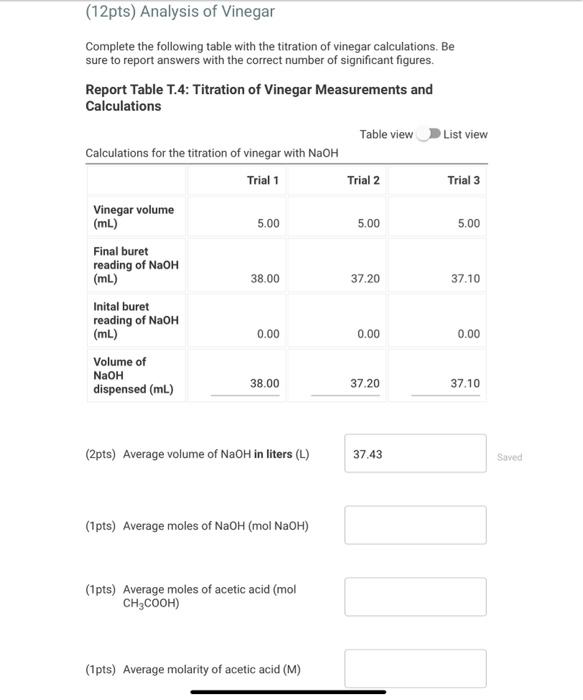

Standardization of a Sodium Hydroxide Solution Note: You may have needed to perform more than three trials to achieve good agreement among three titrations. If you performed more than three titrations, you will only enter the three best trials here. Report Table T.1: Titration of KHC8H4O4 Measurements for the standardization of NaOH Preparing the Vinegar Sample Performing the Titration of Vinegar Note: You may have needed to perform more than three trials to achieve good agreement among three titrations. If you performed more than three titrations, you will only enter the three best trials here. Report Table T.2: Titration of Vinegar Measurements for the titration of vinegar with sodium hyroxide Report Table T.3: Titration of KHC8H4O4 Measurements and Calculations Table view List view Calculations for standardization of sodium hyroxide (1pts) Average molarity of standardized NaOH (M) (12pts) Analysis of Vinegar Complete the following table with the titration of vinegar calculations. Be sure to report answers with the correct number of significant figures. Report Table T.4: Titration of Vinegar Measurements and Calculations Table view List vie Calculations for the titration of vinegar with NaOH (2pts) Average volume of NaOH in liters ( L) (1pts) Average moles of NaOH (mol NaOH) (1pts) Average moles of acetic acid (mol CH3COOH) (1pts) Average molarity of acetic acid ( M ) (1pts) Average mass of acetic acid ( g CH3COOH) (1pts) Average mass of vinegar (g) (2pts) %(m/m)CH3COOH in vinegar (3pts) Percent Error Ask your instructor for the true mass % acetic acid in your vinegar sample. Enter it below, then calculate the percent error for your measured mass \% acetic acid in vinegar. (1pts) True mass \% acetic acid in vinegar (2pts) Percent error (\%)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


