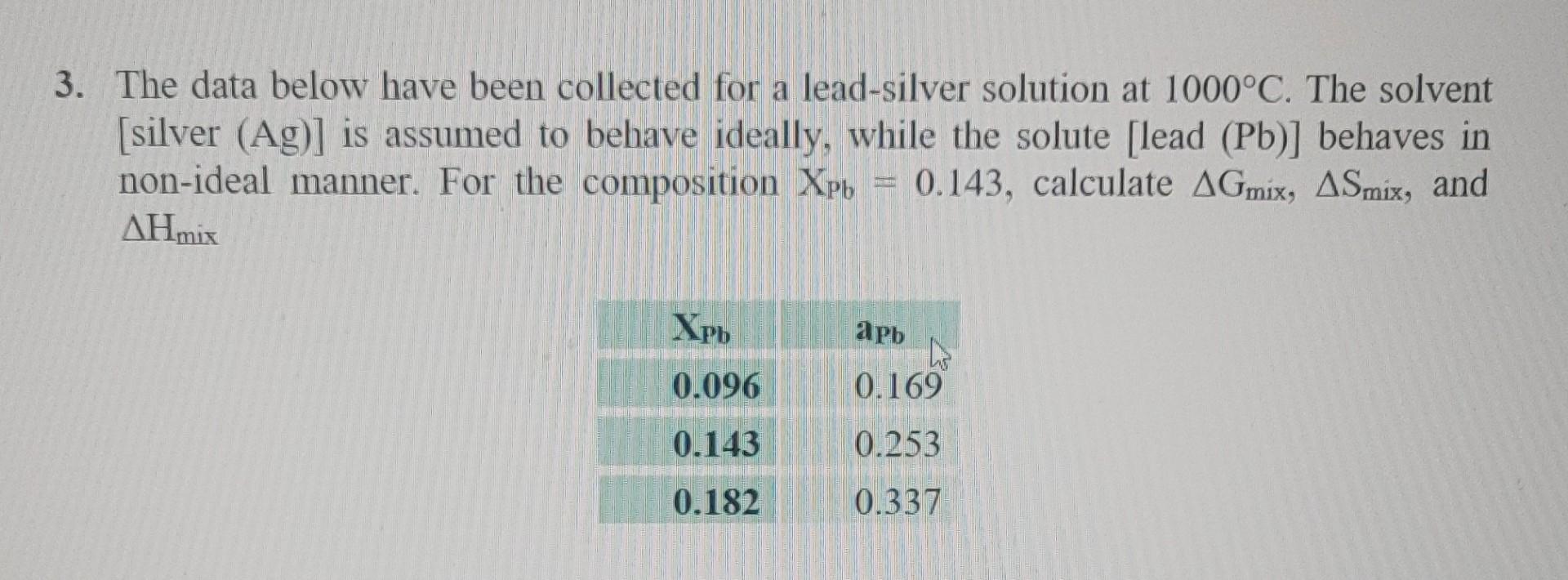
Question: All the information I was given. 3. The data below have been collected for a lead-silver solution at 1000C. The solvent [silver (Ag)] is assumed
All the information I was given.
3. The data below have been collected for a lead-silver solution at 1000C. The solvent [silver (Ag)] is assumed to behave ideally, while the solute [lead (Pb)] behaves in non-ideal manner. For the composition Xpb = 0.143, calculate AGmix, ASmix, and AHmis Xpb apb 0.096 0.169 0.143 0.253 0.182 0.337
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


