Question: All these problems have the same 18 at% Mg but at slightly different temperatures. The equilibrium temperature is the same for all. 1. Analyze the
All these problems have the same 18 at% Mg but at slightly different temperatures. The equilibrium temperature is the same for all.
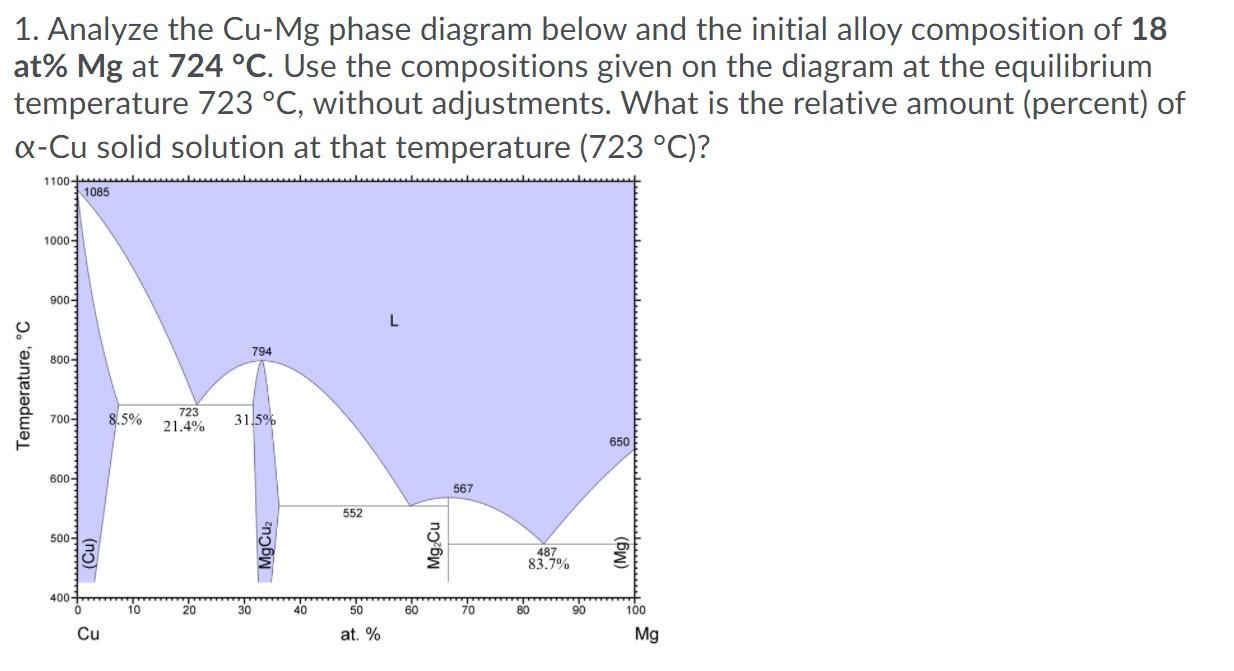
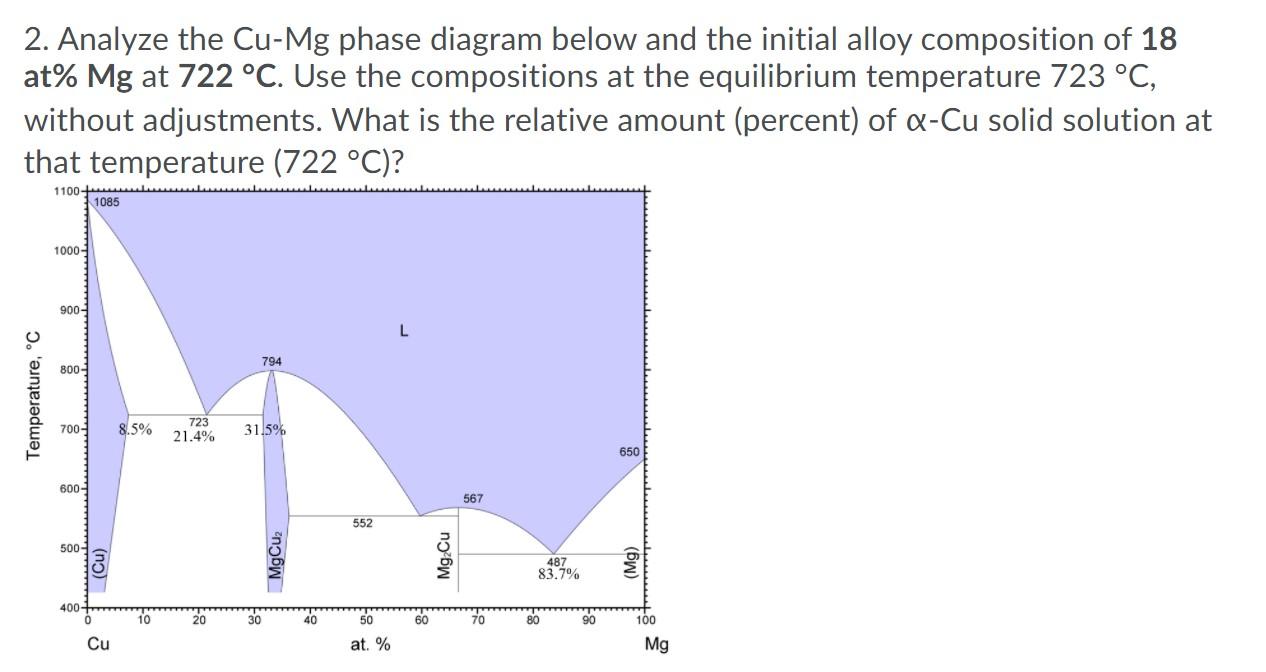
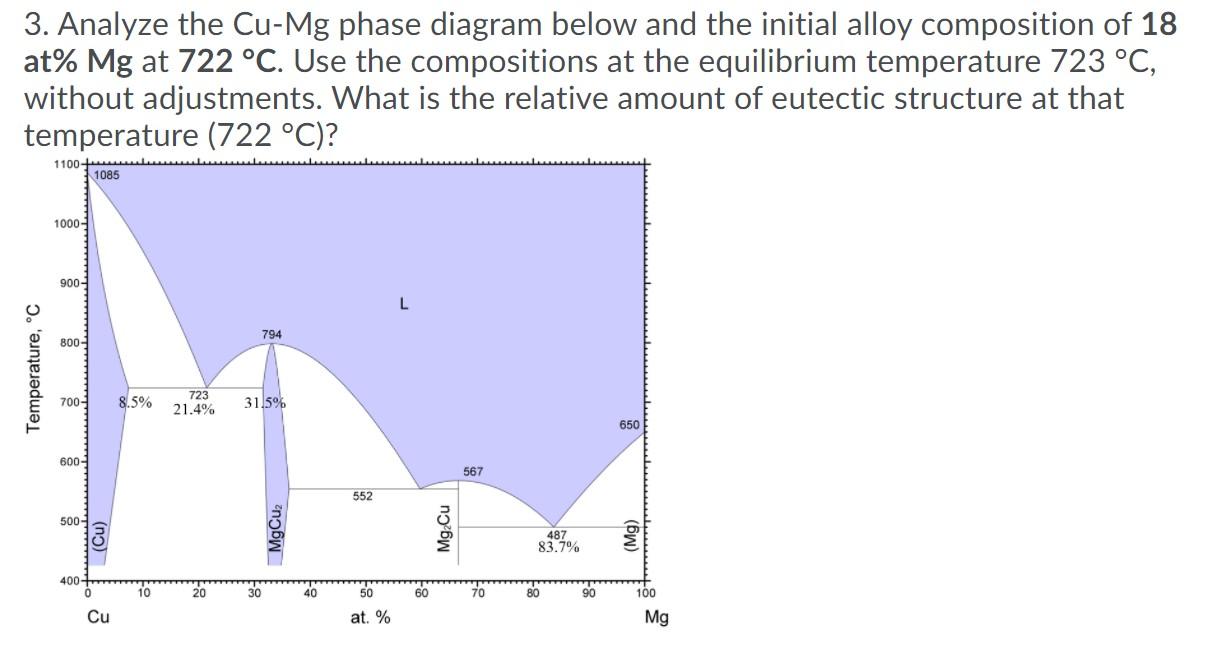
1. Analyze the Cu-Mg phase diagram below and the initial alloy composition of 18 at% Mg at 724 C. Use the compositions given on the diagram at the equilibrium temperature 723 C, without adjustments. What is the relative amount (percent) of Q-Cu solid solution at that temperature (723 C)? 1100- 1085 1000 900- L 794 800- Temperature, C 700- 8.5% 723 21.4% 31.5% 650 600- 567 552 500- (Cu) MgCuz Mg Cu 487 83.7% (Mg) 400 10 20 30 40 50 60 70 80 90 100 Cu at. % Mg 2. Analyze the Cu-Mg phase diagram below and the initial alloy composition of 18 at% Mg at 722 C. Use the compositions at the equilibrium temperature 723 C, without adjustments. What is the relative amount (percent) of Q-Cu solid solution at that temperature (722 C)? 1100- 1085 1000- 900- L 794 800- Temperature, C 700- 8.5% 723 21.4% 31.5% 650 600- 567 552 500- (Cu) MgCuz Mg Cu 487 83.7% (Mg) 400+ 10 30 40 50 60 70 80 90 100 Cu at. % Mg 3. Analyze the Cu-Mg phase diagram below and the initial alloy composition of 18 at% Mg at 722 C. Use the compositions at the equilibrium temperature 723 C, without adjustments. What is the relative amount of eutectic structure at that temperature (722 C)? 1100 1085 1000- 900- L 794 800- Temperature, C 700- 8.5% 723 21.4% 31.5% 650 600- 567 552 500 (Cu) MgCuz Mg Cu 487 83.7% (Mg) 400 10 20 30 40 50 60 70 80 100 Cu at. % Mg
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


