Question: also compute deltaH for reaction (3) Use the data at the back of the textbook (also on the PPT) to determine H for the process

also compute deltaH for reaction
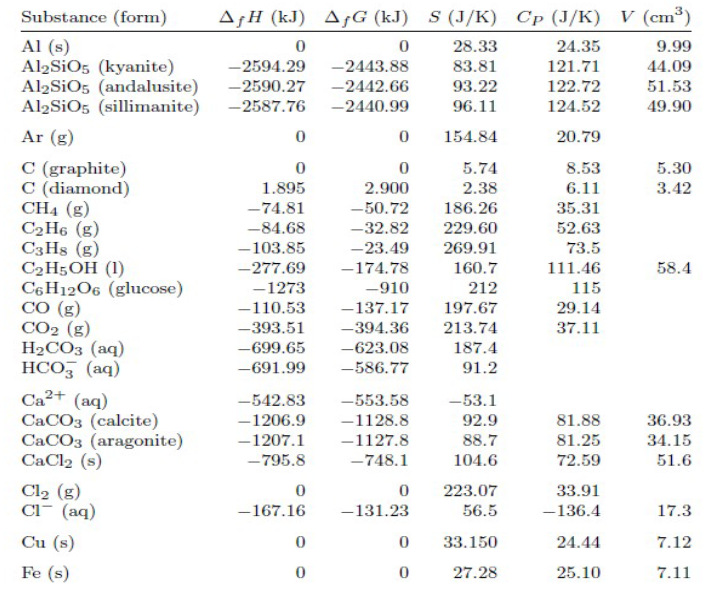
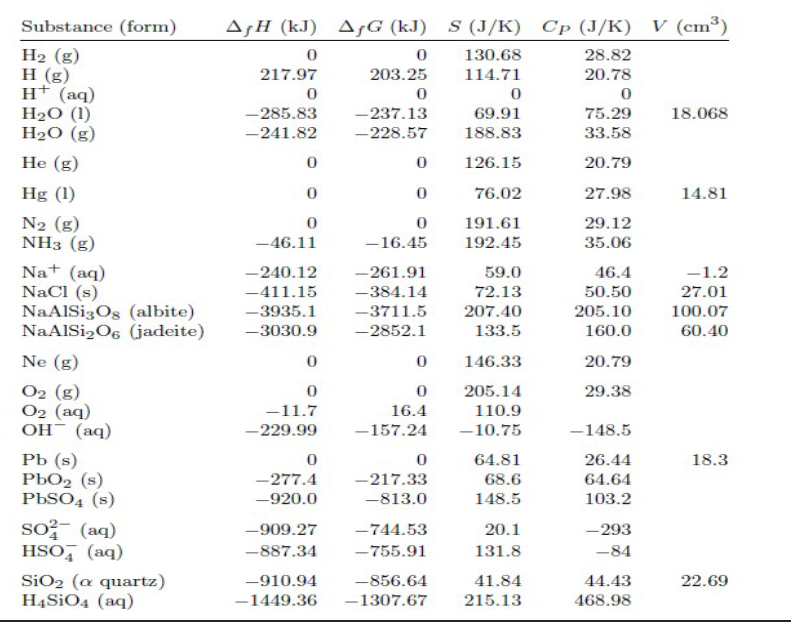
(3) Use the data at the back of the textbook (also on the PPT) to determine H for the process shown below. The product is liquid water. C6H12O6+6O26CO2+6H2O. b) Now assume that the product is water vapor and determine how much work is heeded to be done in order to "create room" for the products? (HINT: You need to ompute a new H )
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


