Question: also list all atom positions for iv) do the calculation Question 3 (i) Draw a face-centered cubic (FCC) unit cell showing atoms and list out
also list all atom positions
for iv) do the calculation
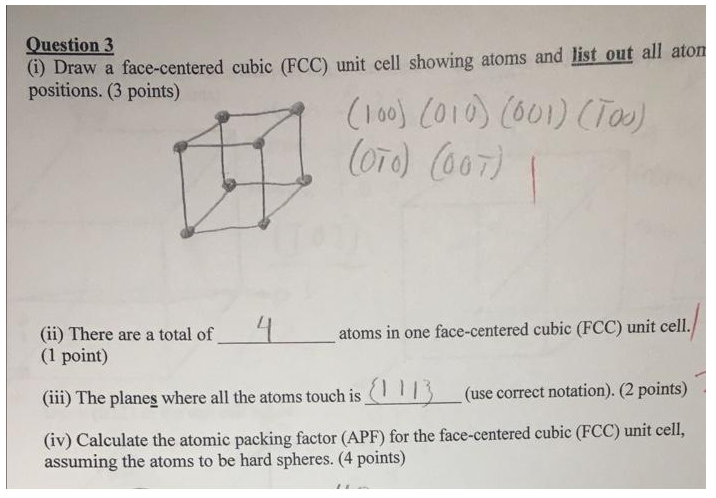
Question 3 (i) Draw a face-centered cubic (FCC) unit cell showing atoms and list out all aton positions. (3 points) (100) (010) (601) (TO) (OTO) (OOT) ( (ii) There are a total of 4 atoms in one face-centered cubic (FCC) unit cell. (1 point) (iii) The planes where all the atoms touch is 1113_(use correct notation). (2 points) (iv) Calculate the atomic packing factor (APF) for the face-centered cubic (FCC) unit cell, assuming the atoms to be hard spheres. (4 points)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


