Question: Aluminum is anodized for 8 hours using a constant applied potential. A current is measured at each hour and recorded in the table below. Assuming
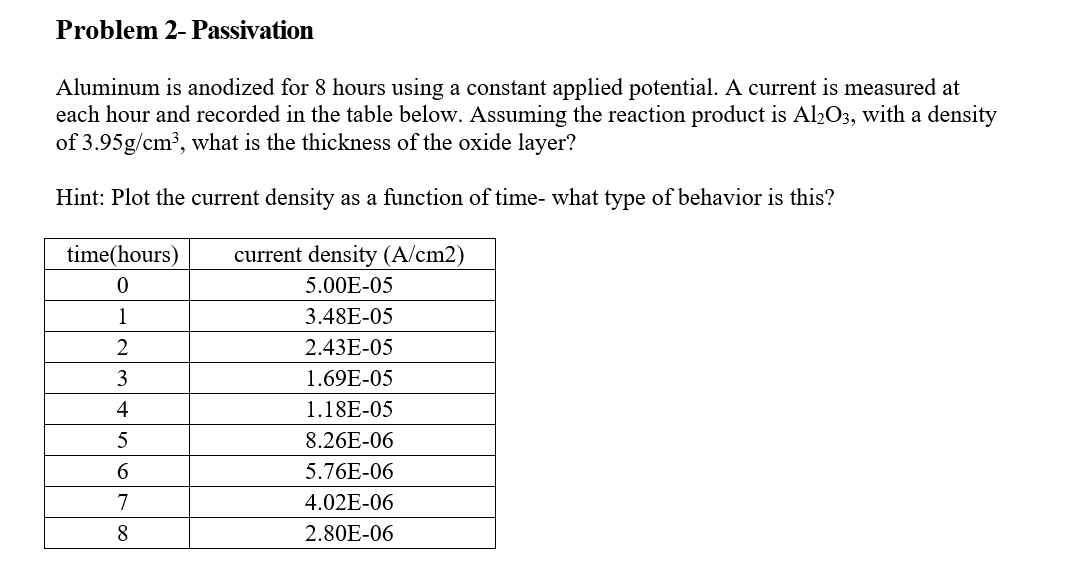
Aluminum is anodized for 8 hours using a constant applied potential. A current is measured at each hour and recorded in the table below. Assuming the reaction product is Al2O3, with a density of 3.95g/cm3, what is the thickness of the oxide layer? Hint: Plot the current density as a function of time- what type of behavior is this
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


