Question: Ammonia is absorbed from a gas stream into water using a plate column. The column operates at 2 bar and 2 2 C . The
Ammonia is absorbed from a gas stream into water using a plate column. The column operates at bar and The gas enters at a rate of kmol per hour containing
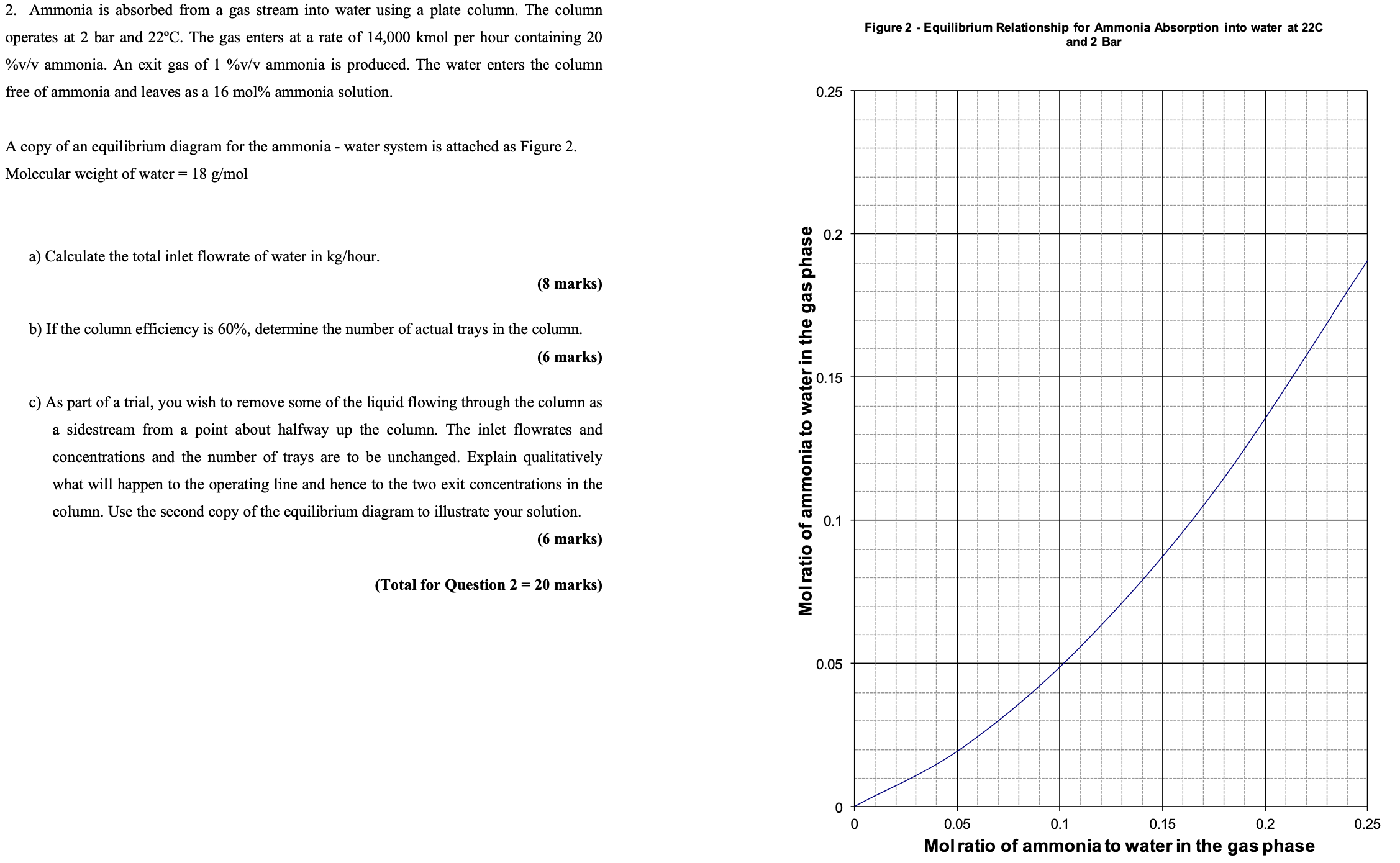
Figure Equilibrium Relationship for Ammonia Absorption into water at
and Bar
ammonia. An exit gas of ammonia is produced. The water enters the column free of ammonia and leaves as a mol ammonia solution.
A copy of an equilibrium diagram for the ammonia water system is attached as Figure
Molecular weight of water
a Calculate the total inlet flowrate of water in our.
marks
b If the column efficiency is determine the number of actual trays in the column.
marks
c As part of a trial, you wish to remove some of the liquid flowing through the column as a sidestream from a point about halfway up the column. The inlet flowrates and concentrations and the number of trays are to be unchanged. Explain qualitatively what will happen to the operating line and hence to the two exit concentrations in the column. Use the second copy of the equilibrium diagram to illustrate your solution.
marks
Total for Question marks
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


