Question: Ammonia, NH3, is a weak base with a Kb value of 1.8105. The degree to which a weak base dissociates is given by the base-ionization
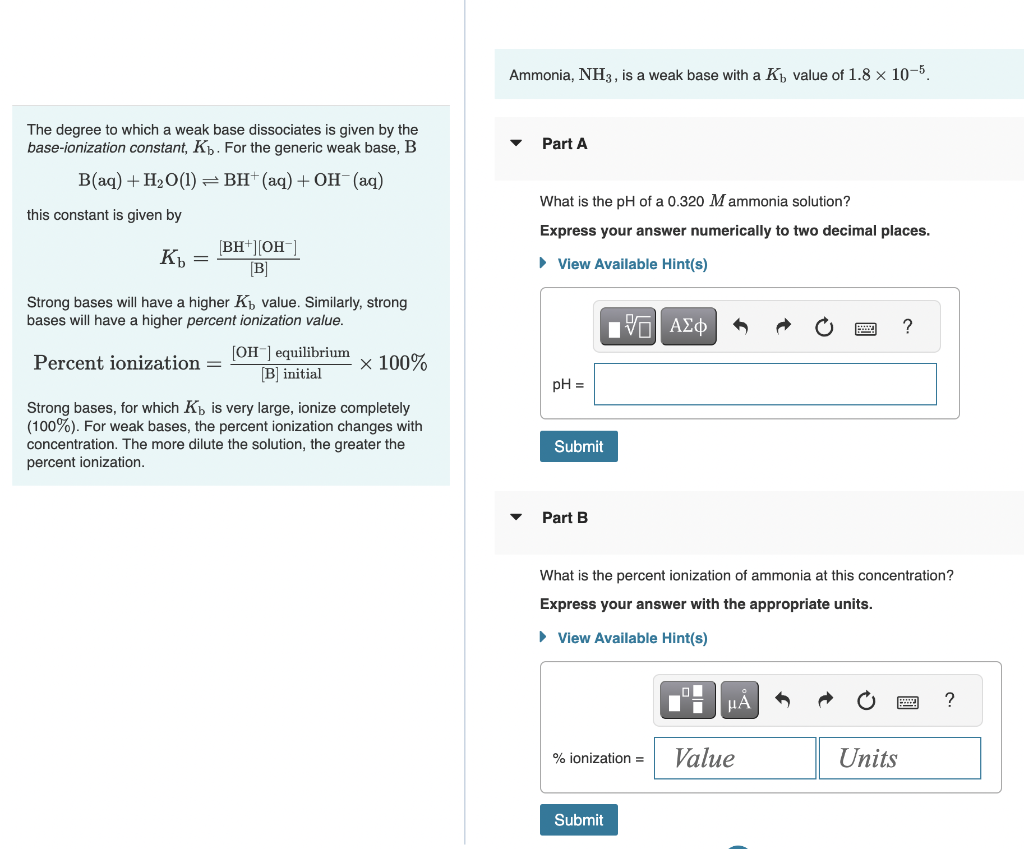
Ammonia, NH3, is a weak base with a Kb value of 1.8105. The degree to which a weak base dissociates is given by the base-ionization constant, Kb. For the generic weak base, B Part A B(aq)+H2O(l)BH+(aq)+OH(aq) What is the pH of a 0.320M ammonia solution? this constant is given by Express your answer numerically to two decimal places. Kb=[B][BH+][OH] Strong bases will have a higher Kb value. Similarly, strong bases will have a higher percent ionization value. Percentionization=[B]initial[OH]equilibrium100% Strong bases, for which Kb is very large, ionize completely (100%). For weak bases, the percent ionization changes with concentration. The more dilute the solution, the greater the percent ionization. Part B What is the percent ionization of ammonia at this concentration? Express your answer with the appropriate units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


