Question: Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studying this reaction fills a 2.0 I. flask with 0.67 atm of
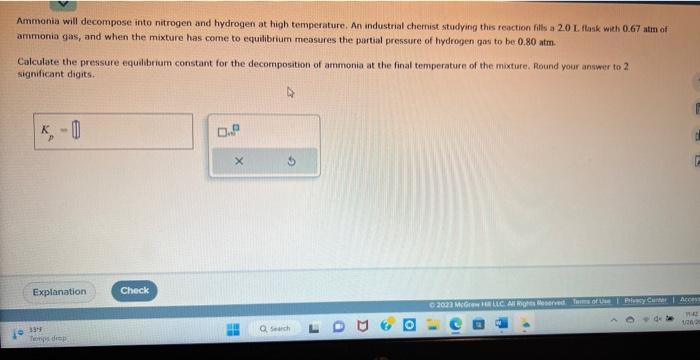
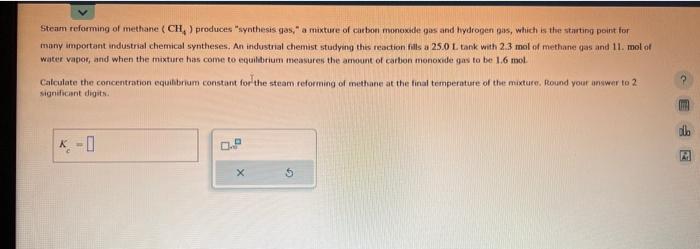
Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studying this reaction fills a 2.0 I. flask with 0.67 atm of ammonia gas, and when the mixture has come to equilibrium measures the partal pressure of hydrogen gas to be- 0.80 atm. Calculate the pressure equibbrium constant for the decomposition of ammonia at the final temperature of the mixture, Round your answer to 2 . significant digits. Steam reforming of methane (CH4) produces "synthesis gas," a maxtare of carboe monoxide pas and hydropen gas, which is the starting peint for many important industrial chemical syntheses. An industrial chemist studying this reaction fals a 2.5.0L. tank with 2.3 mol of methane qas and 11 . mol of water vapog, and when the mixture has come to equilbrium measures the ariount of carbon monoxide gas to be 1.6 mol. Calculate the concentration equibbrium constant for the steam reforming of methane at the final temperature of the mixture. Round your answer to 2 significint digits
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


