Question: Ammonium perchlorate (NH4ClO4) is a powerful solid rocket fuel, used in the Space Shuttle boosters. It decomposes into ntrogen ( N2 ) gas, chiorine (Cl
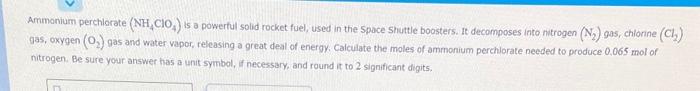
Ammonium perchlorate (NH4ClO4) is a powerful solid rocket fuel, used in the Space Shuttle boosters. It decomposes into ntrogen ( N2 ) gas, chiorine (Cl ) 9a5, oxygen (O2) gas and water vapor, releasing a great deal of energy. Calculate the moles of ammonium perchlorate needed to produce 0.065 mol of nitrogen. Be sure your answer has a unit syimbol, if necessary, and round it to 2 significant digits
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


