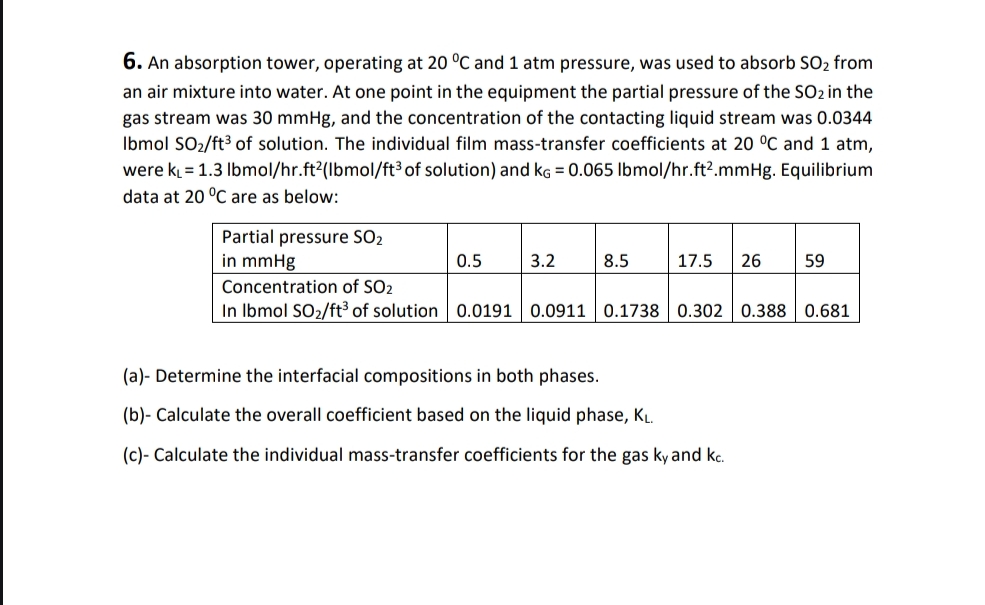
Question: An absorption tower, operating at 2 0 C and 1 atm pressure, was used to absorb S O 2 from an air mixture into water.
An absorption tower, operating at and atm pressure, was used to absorb from an air mixture into water. At one point in the equipment the partial pressure of the in the gas stream was and the concentration of the contacting liquid stream was IbmolS of solution. The individual film masstransfer coefficients at and atm, were of solution and lbmo Equilibrium data at are as below:
tabletablePartial pressure
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


