Question: An azeotrope was formed for a binary liquid mixture at 140 C with the azeotrope composition x^2 = yaz 0.294. The saturated pressure for components
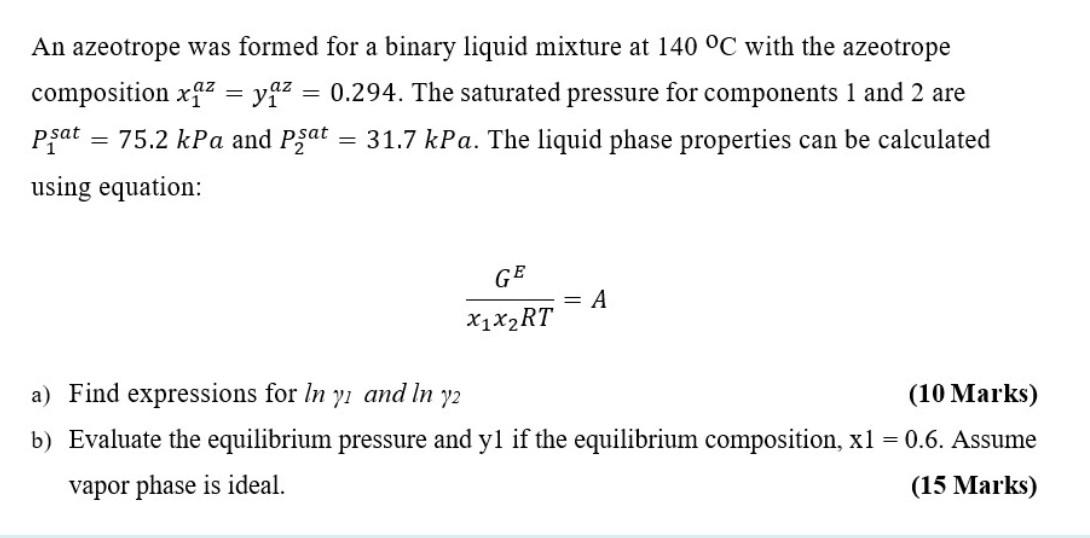
An azeotrope was formed for a binary liquid mixture at 140 C with the azeotrope composition x^2 = yaz 0.294. The saturated pressure for components 1 and 2 are psat = 75.2 kPa and Pat 31.7 kPa. The liquid phase properties can be calculated using equation: GE = A X1X2RT a) Find expressions for In y and In y2 (10 Marks) b) Evaluate the equilibrium pressure and yl if the equilibrium composition, x1 = 0.6. Assume vapor phase is ideal. (15 Marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


