Question: An easy alternative to working with this dH equation is to use residuals, and so far, the primary way we calculated residuals was using the
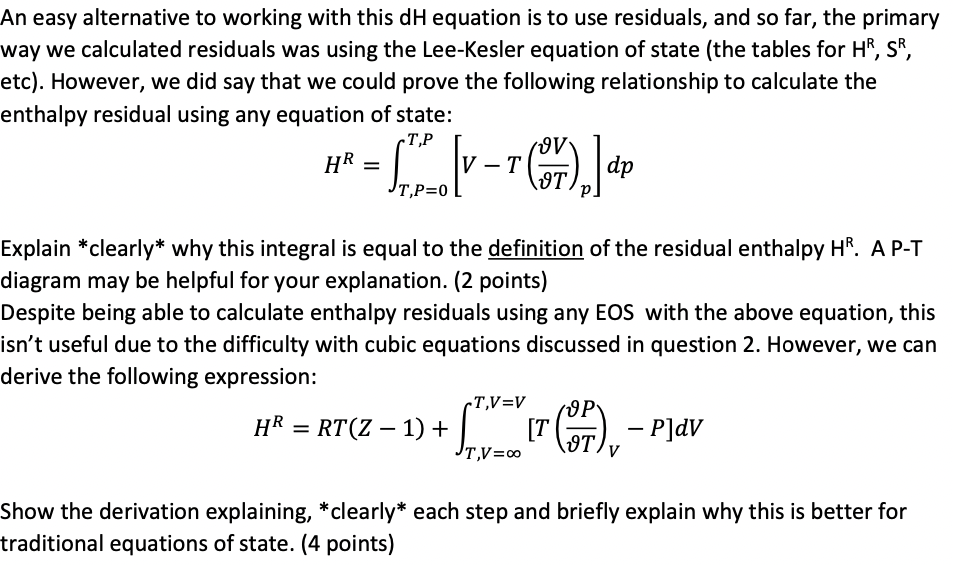
An easy alternative to working with this dH equation is to use residuals, and so far, the primary way we calculated residuals was using the Lee-Kesler equation of state (the tables for HR,SR, etc). However, we did say that we could prove the following relationship to calculate the enthalpy residual using any equation of state: HR=T,P=0T,P[VT(TV)p]dp Explain *clearly* why this integral is equal to the definition of the residual enthalpy HR. A P-T diagram may be helpful for your explanation. (2 points) Despite being able to calculate enthalpy residuals using any EOS with the above equation, this isn't useful due to the difficulty with cubic equations discussed in question 2. However, we can derive the following expression: HR=RT(Z1)+T,V=T,V=V[T(TP)VP]dV Show the derivation explaining, *clearly* each step and briefly explain why this is better for traditional equations of state. (4 points)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


