Question: An electron has the quantum numbers n = 3 , l = 2 , m l = 1 , m s = + 1 2
An electron has the quantum numbers In which orbital is this electron
found?
a
c
e
b
d
f
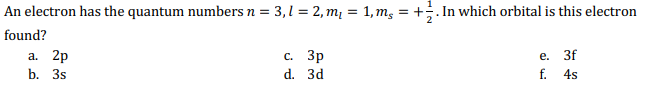
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


