Question: An equilibrium system is established in a 1 . 0 0 L flask at 8 0 0 C , as represented by the following equation:
An equilibrium system is established in a flask at as represented by the following equation:
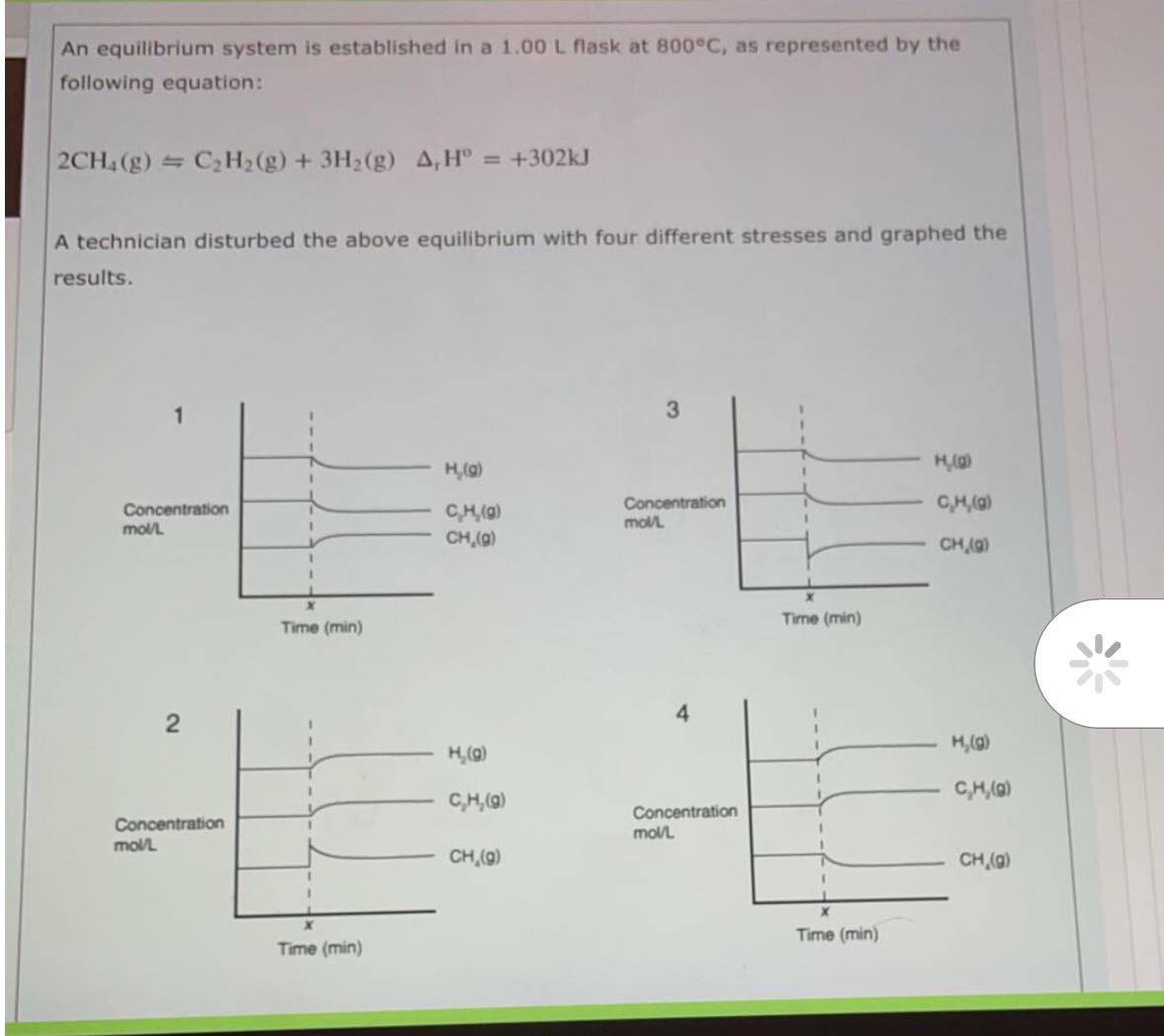
A technician disturbed the above equilibrium with four different stresses and graphed the results. Match the graphs with the stresses listed
system was cooled added CHg removed CHg decrease the pressure
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


