Question: An object was a neutral and then gains a certain number of electrons and the net charge is now -5.6 x 10-14 C. Determine
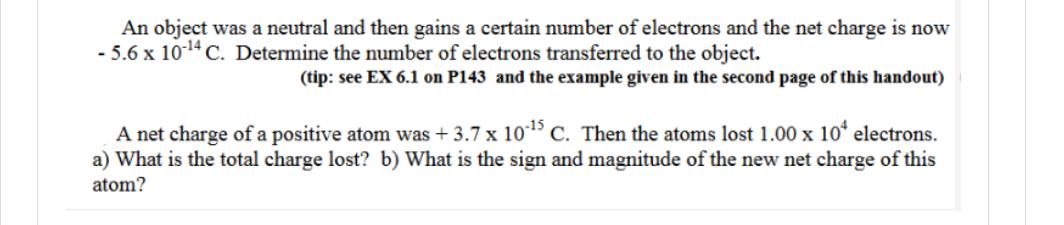
An object was a neutral and then gains a certain number of electrons and the net charge is now -5.6 x 10-14 C. Determine the number of electrons transferred to the object. (tip: see EX 6.1 on P143 and the example given in the second page of this handout) A net charge of a positive atom was +3.7 x 10-15 C. Then the atoms lost 1.00 x 10 electrons. a) What is the total charge lost? b) What is the sign and magnitude of the new net charge of this atom?
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


