Question: An object was weighed by 5 different students with the following results: student 1: 45mg student 2: 47mg student 3:38mg student 4: 40mg student 5:
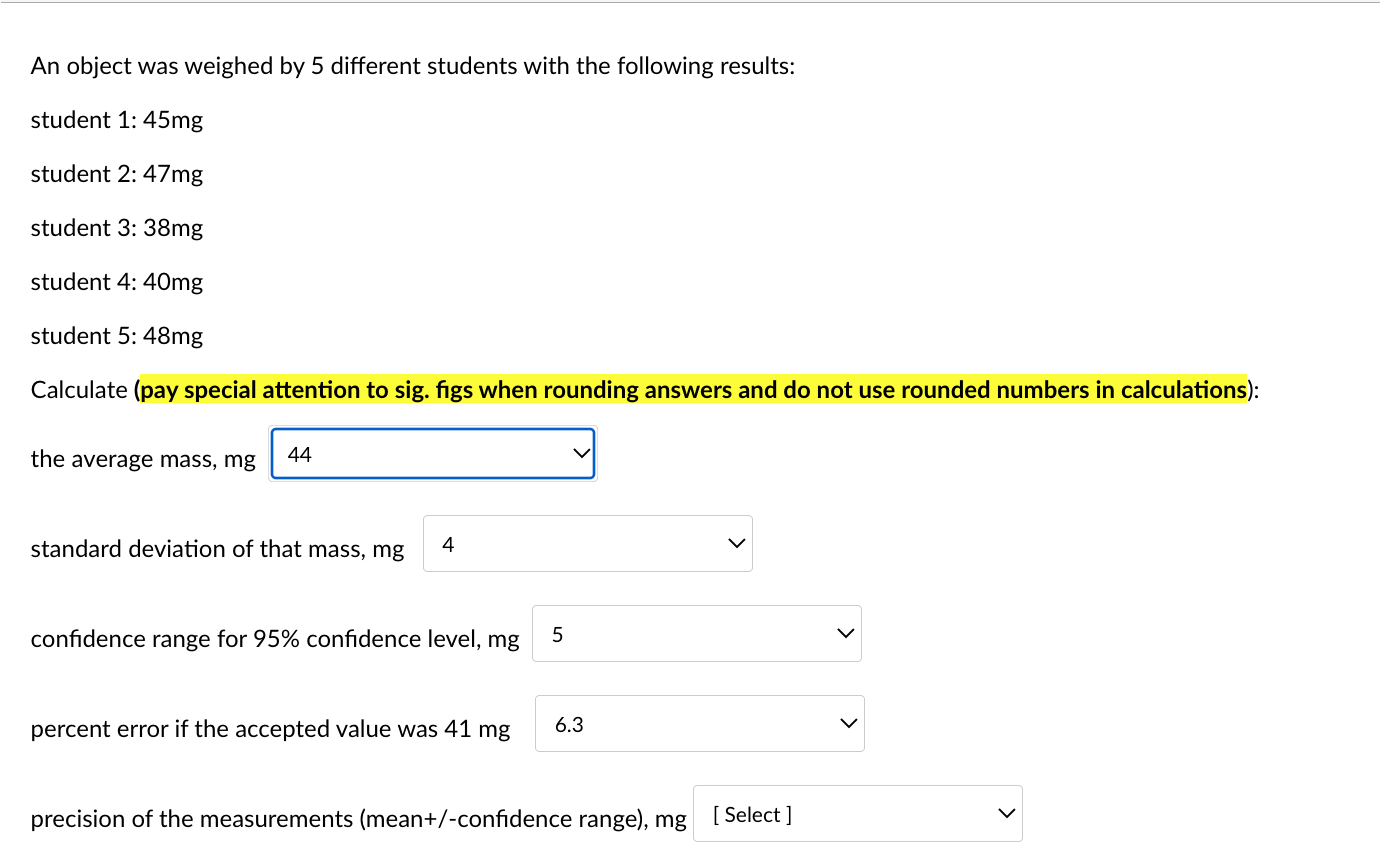
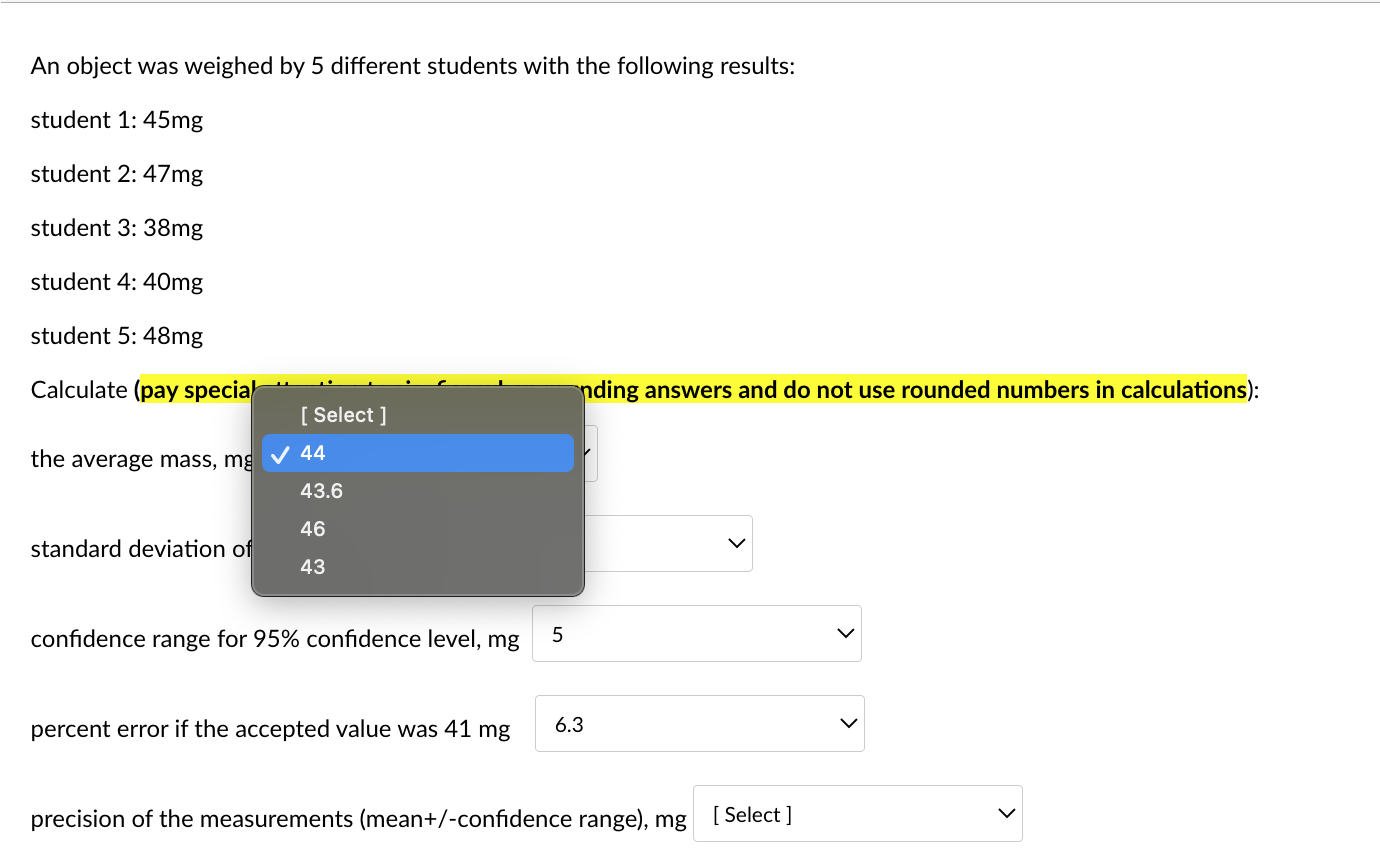
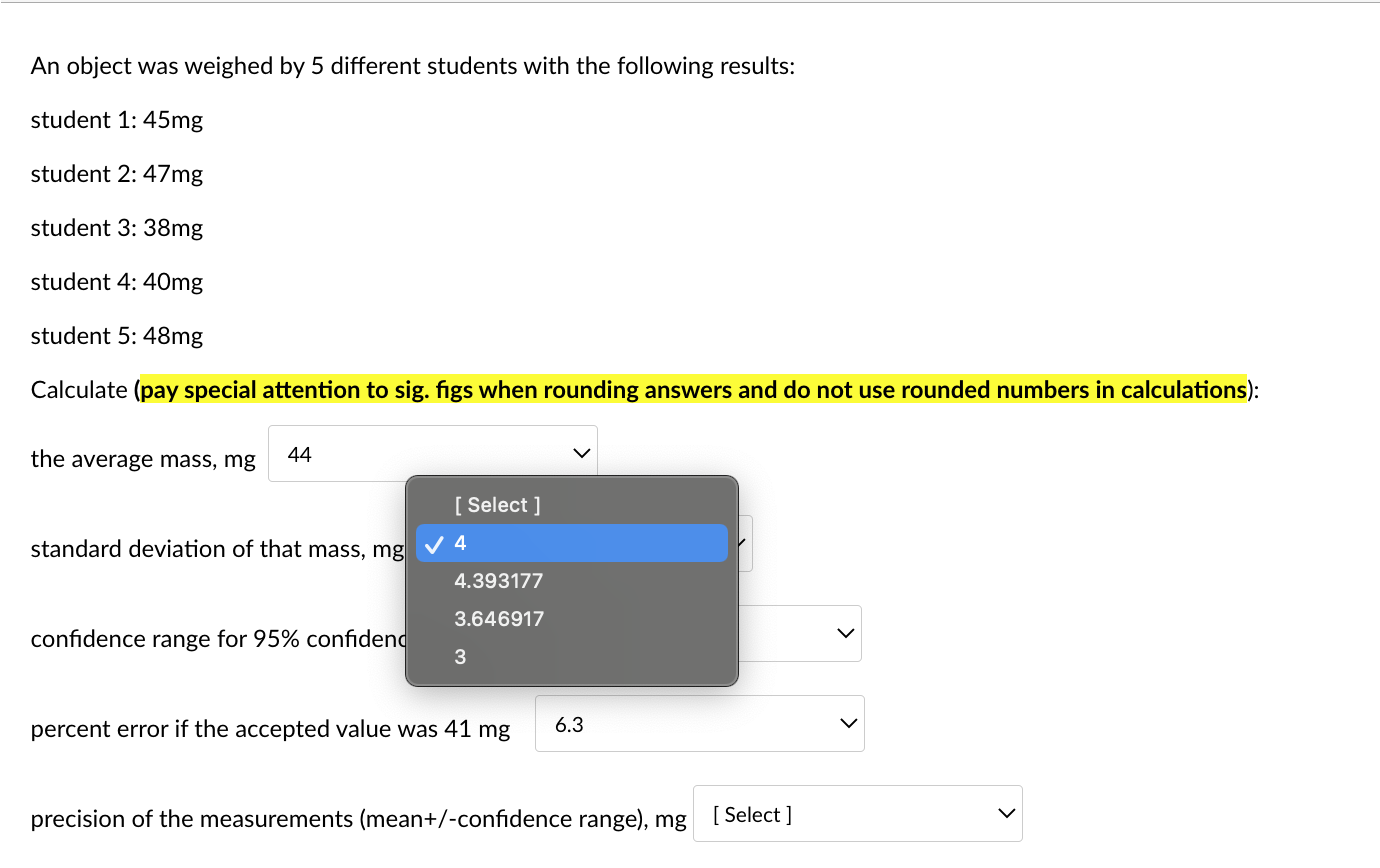
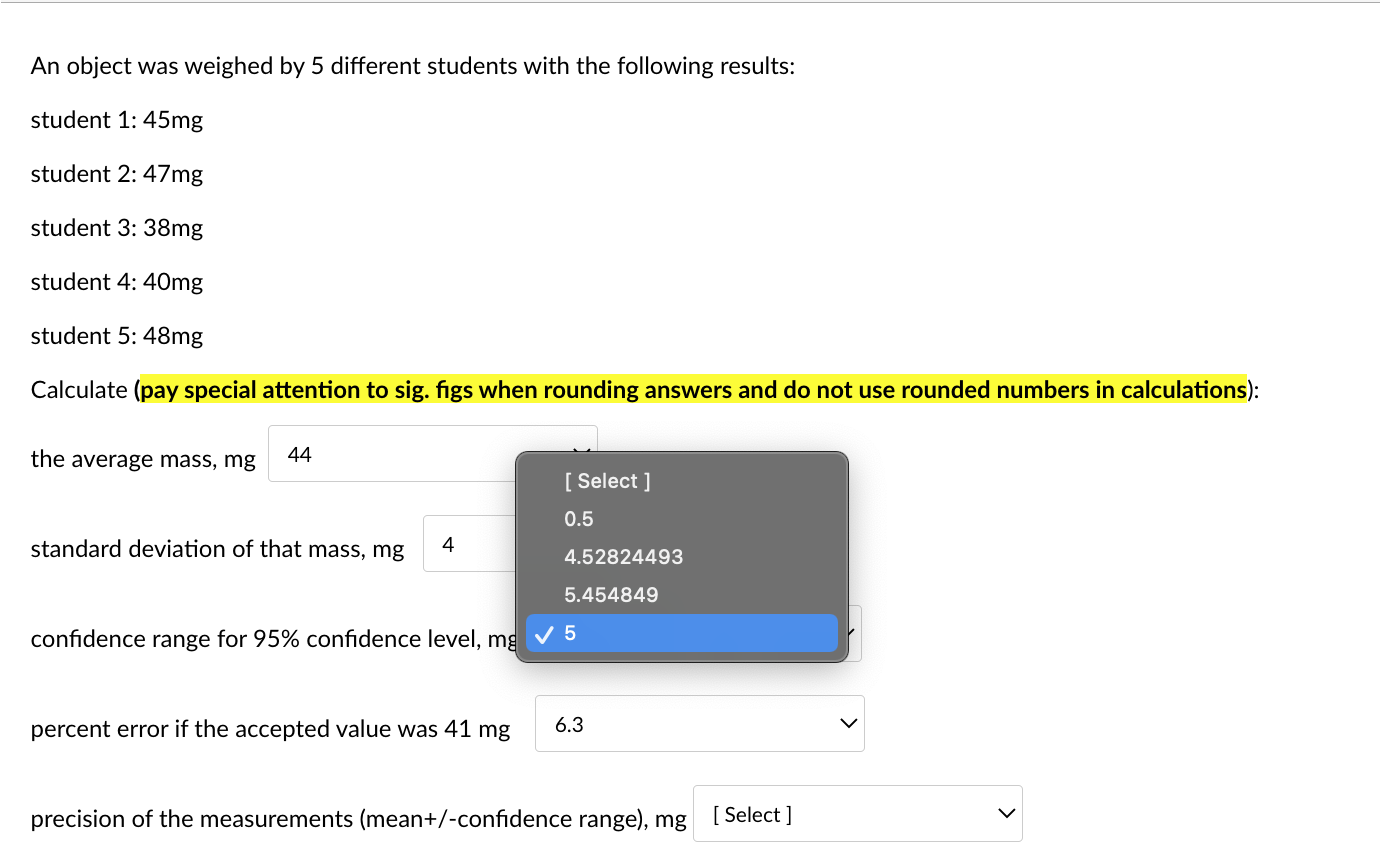
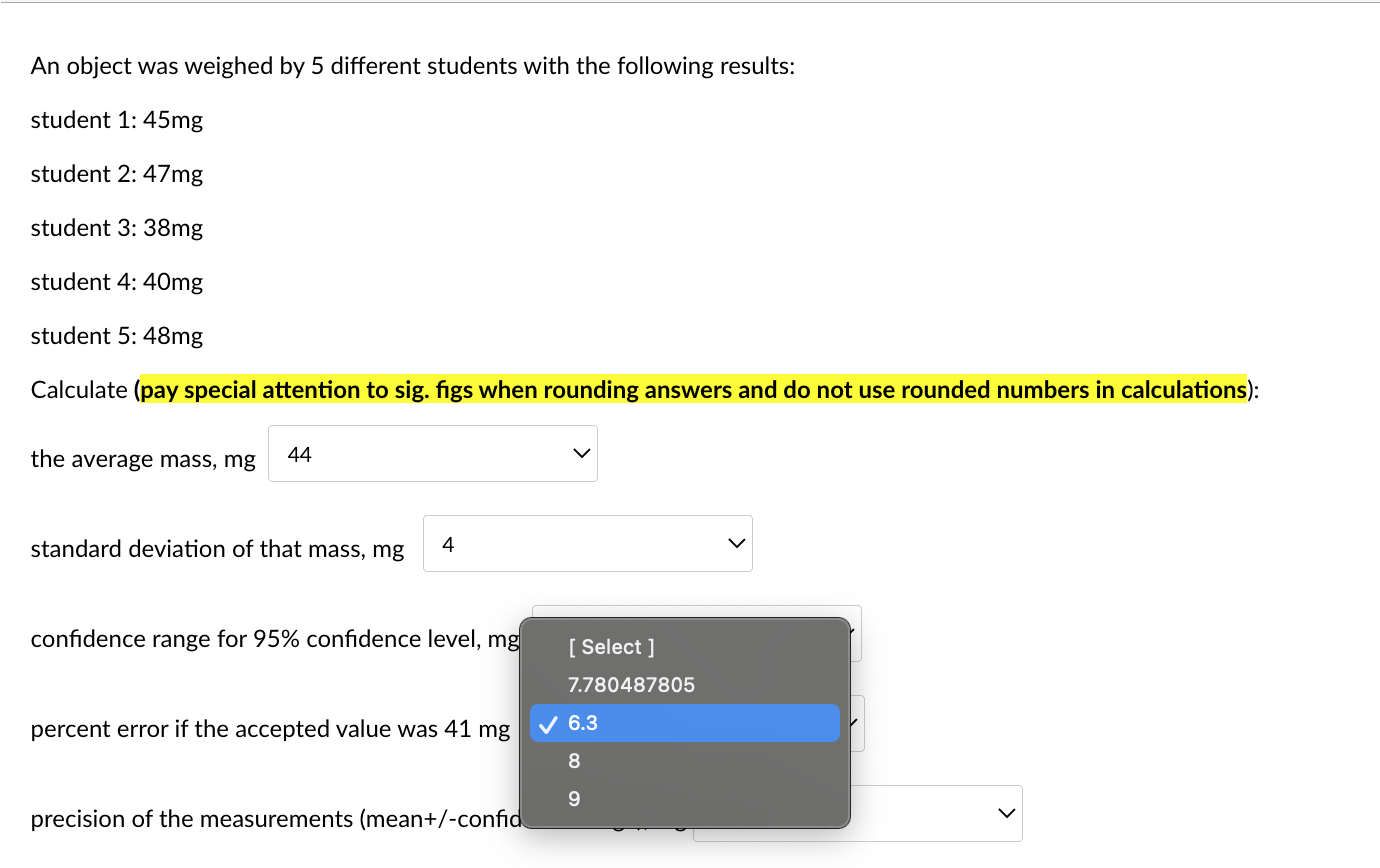
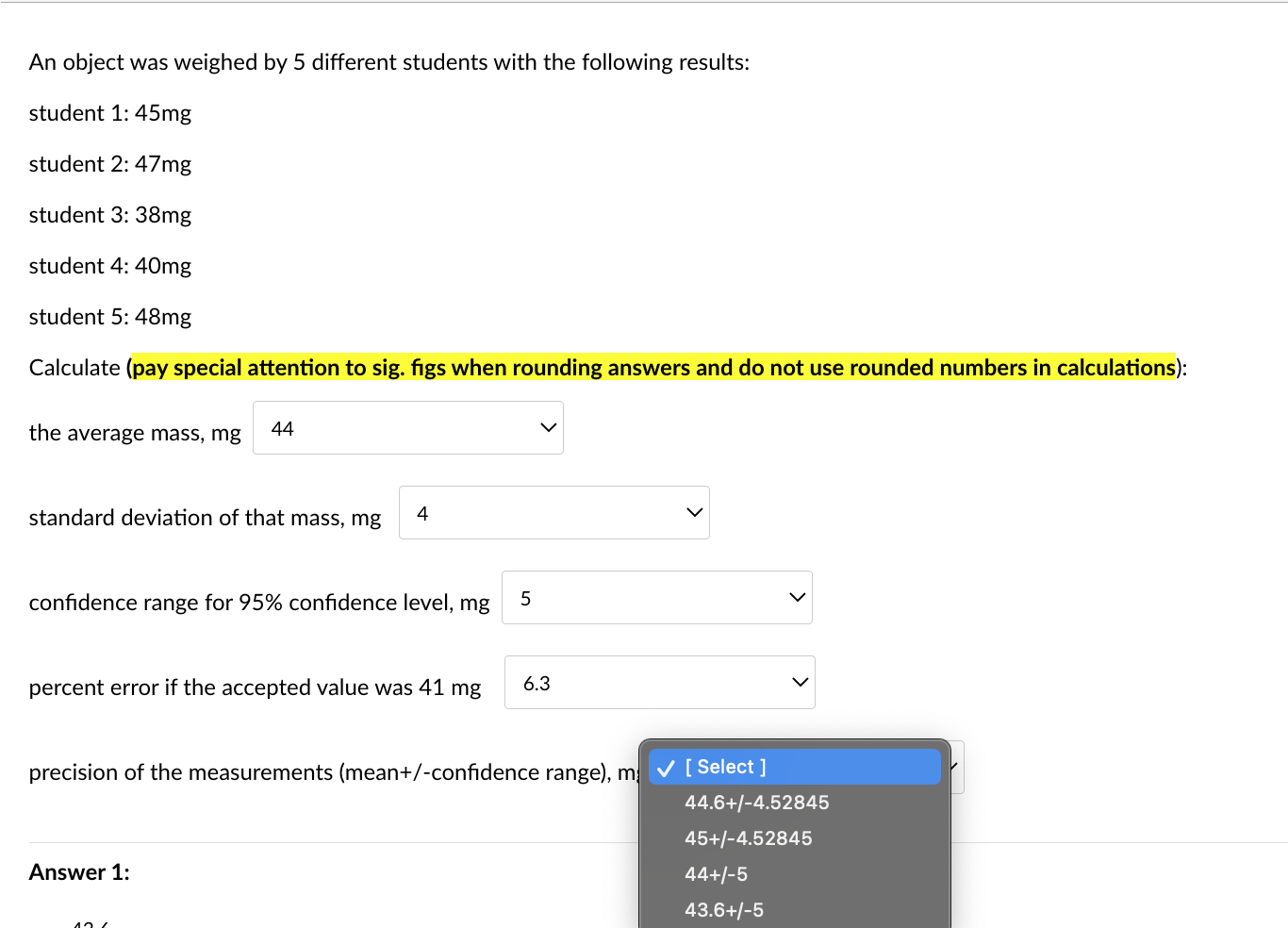
An object was weighed by 5 different students with the following results: student 1: 45mg student 2: 47mg student 3:38mg student 4: 40mg student 5: 48mg Calculate (pay special attention to sig. figs when rounding answers and do not use rounded numbers in calculations): the average mass, mg standard deviation of that mass, mg confidence range for 95% confidence level, mg percent error if the accepted value was 41mg precision of the measurements (mean +/-confidence range), mg An object was weighed by 5 different students with the following results: student 1: 45mg student 2: 47mg student 3: 38mg student 4: 40mg student 5: 48mg \begin{tabular}{c|c} the average mass, m& & Selec 44 43.6 46 \\ standard deviation of & 43 \end{tabular} confidence range for 95% confidence level, mg 5 percent error if the accepted value was 41mg 6.3 precision of the measurements (mean+/-confidence range), mg [ Select ] An object was weighed by 5 different students with the following results: student 1: 45mg student 2: 47mg student 3: 38mg student 4: 40mg student 5: 48mg Calculate (pay special attention to sig. figs when rounding answers and do not use rounded numbers in calculations): the average mass, mg standard deviation of that mass, mg confidence range for 95% confidenc percent error if the accepted value was 41mg precision of the measurements (mean+/-confidence range), mg An object was weighed by 5 different students with the following results: student 1: 45mg student 2: 47mg student 3: 38mg student 4: 40mg student 5: 48mg Calculate (pay special attention to sig. figs when rounding answers and do not use rounded numbers in calculations): the average mass, mg standard deviation of that mass, mg confidence range for 95% confidence level, mg percent error if the accepted value was 41mg precision of the measurements (mean+/-confidence range), mg An object was weighed by 5 different students with the following results: student 1: 45mg student 2: 47mg student 3: 38mg student 4: 40mg student 5: 48mg Calculate (pay special attention to sig. figs when rounding answers and do not use rounded numbers in calculations): the average mass, mg standard deviation of that mass, mg confidence range for 95% confidence level, mg percent error if the accepted value was 41mg precision of the measurements (mean+/-confic An object was weighed by 5 different students with the following results: student 1: 45mg student 2: 47mg student 3: 38mg student 4: 40mg student 5: 48mg Calculate (pay special attention to sig. figs when rounding answers and do not use rounded numbers in calculations): the average mass, mg standard deviation of that mass, mg confidence range for 95% confidence level, mg percent error if the accepted value was 41mg precision of the measurements (mean + /-confidence range), m
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


