Question: An Problem 6 ( 2 5 points ) An ideal gas ) = ( 2 8 . 3 1 4 J m o l K
An Problem points
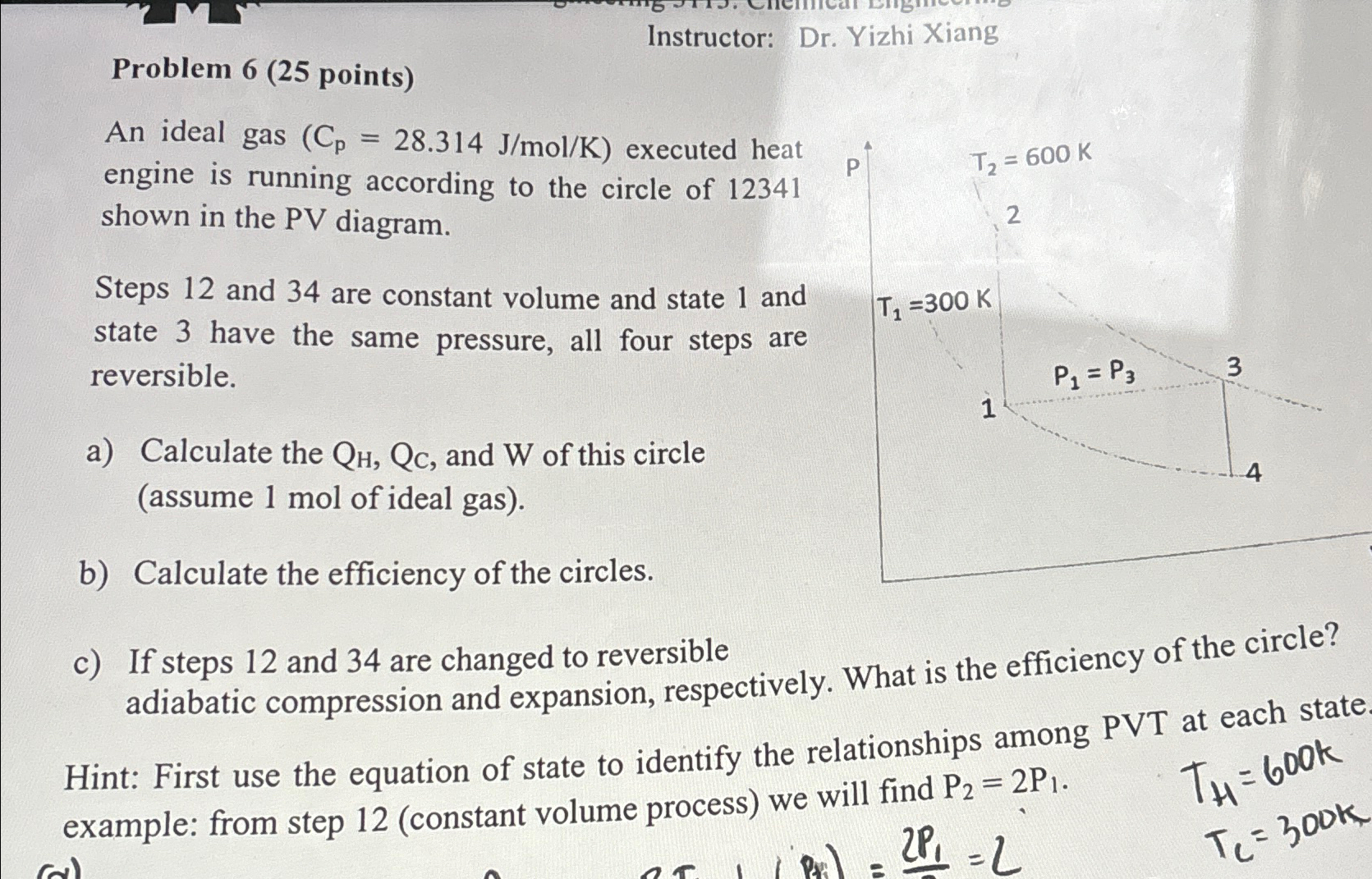
An ideal gas executed heat engine is running according to the circle of shown in the PV diagram.
Steps and are constant volume and state and state have the same pressure, all four steps are reversible.
a Calculate the and of this circle assume mol of ideal gas
b Calculate the efficiency of the circles.
c If steps and are changed to reversible adiabatic compression and expansion, respectively. What is the efficiency of the circle?
Hint: First use the equation of state to identify the relationships among PVT at each state example: from step constant volume process we will find
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


