Question: And use the page below (pg. 91) to answer the questions for the page above (pg. 103) Name: Date: TA: Section: Answer the following questions

And use the page below (pg. 91) to answer the questions for the page above (pg. 103)
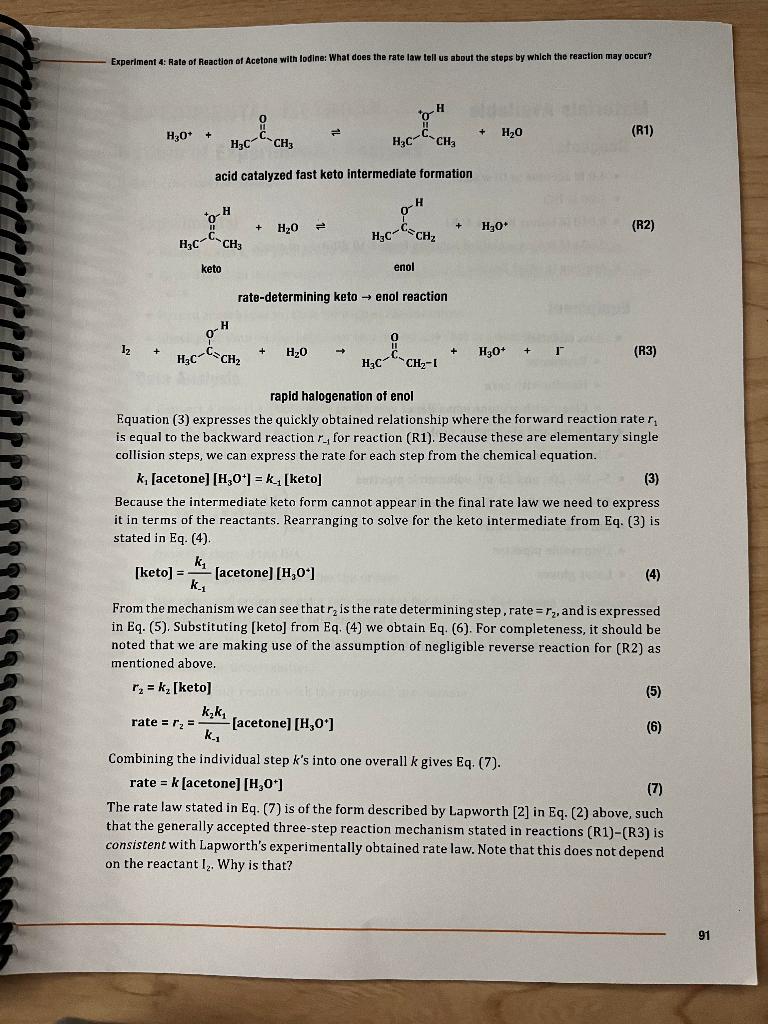
Name: Date: TA: Section: Answer the following questions using information obtained from lecture, the textbook, or lab manual. The responses will be collected at the beginning of recitation and checked at the start of your lab section for credit. 1. Understanding the relationship between a proposed mechanism and the experimental rate law is crucial to interpreting the chemistry underlying the chemical reaction. The proposed mechanism for this reaction is presented in (R1)-(R3), see page 91. Describe an analogy (real life situation) that fits the mechanism and explain the connection. In other words, we are looking for an experience you have had where there was something that limited how fast things could happen. Before and after that step, things could move fast. a. What situations have you encountered with a rate determining step? You may want to include several examples to be able to choose from below. b. Now, can you think of a rate determining process example that has a fast forward and backward process before it? c. Describe the fast forward process that would occur before your rate determining step. d. Describe the fast backward process that occurs before your rate determining step. e. Describe the fast non rate determining process that occurs after the rate limiting step in your analysis. Experiment 4: Rate of Reaction of Acetone with lodine: What does the rate law lell us about the stops by which the reaction may occer? (R1) acid catalyzed fast keto intermediate formation keto enol rate-determining keto enol reaction (R2) rapid halogenation of enol Equation (3) expresses the quickly obtained relationship where the forward reaction rate r1 is equal to the backward reaction r1 for reaction (R1). Because these are elementary single collision steps, we can express the rate for each step from the chemical equation. k1[acetone][H3O+]=k1[keto] Because the intermediate keto form cannot appear in the final rate law we need to express it in terms of the reactants. Rearranging to solve for the keto intermediate from Eq. (3) is stated in Eq. (4). [keto] =k1k1[ acetone ][H3O+] From the mechanism we can see that r2 is the rate determining step, rate =r2, and is expressed in Eq. (5). Substituting [keto] from Eq. (4) we obtain Eq. (6). For completeness, it should be noted that we are making use of the assumption of negligible reverse reaction for (R2) as mentioned above. r2=k2[keto]rate=r2=k1k2k1[acetone[H3O+] Combining the individual step k 's into one overall k gives Eq. (7). rate=k[acetone][H3O+] The rate law stated in Eq. (7) is of the form described by Lapworth [2] in Eq. (2) above, such that the generally accepted three-step reaction mechanism stated in reactions (R1)(R3) is consistent with Lapworth's experimentally obtained rate law. Note that this does not depend on the reactant I2. Why is that? Name: Date: TA: Section: Answer the following questions using information obtained from lecture, the textbook, or lab manual. The responses will be collected at the beginning of recitation and checked at the start of your lab section for credit. 1. Understanding the relationship between a proposed mechanism and the experimental rate law is crucial to interpreting the chemistry underlying the chemical reaction. The proposed mechanism for this reaction is presented in (R1)-(R3), see page 91. Describe an analogy (real life situation) that fits the mechanism and explain the connection. In other words, we are looking for an experience you have had where there was something that limited how fast things could happen. Before and after that step, things could move fast. a. What situations have you encountered with a rate determining step? You may want to include several examples to be able to choose from below. b. Now, can you think of a rate determining process example that has a fast forward and backward process before it? c. Describe the fast forward process that would occur before your rate determining step. d. Describe the fast backward process that occurs before your rate determining step. e. Describe the fast non rate determining process that occurs after the rate limiting step in your analysis. Experiment 4: Rate of Reaction of Acetone with lodine: What does the rate law lell us about the stops by which the reaction may occer? (R1) acid catalyzed fast keto intermediate formation keto enol rate-determining keto enol reaction (R2) rapid halogenation of enol Equation (3) expresses the quickly obtained relationship where the forward reaction rate r1 is equal to the backward reaction r1 for reaction (R1). Because these are elementary single collision steps, we can express the rate for each step from the chemical equation. k1[acetone][H3O+]=k1[keto] Because the intermediate keto form cannot appear in the final rate law we need to express it in terms of the reactants. Rearranging to solve for the keto intermediate from Eq. (3) is stated in Eq. (4). [keto] =k1k1[ acetone ][H3O+] From the mechanism we can see that r2 is the rate determining step, rate =r2, and is expressed in Eq. (5). Substituting [keto] from Eq. (4) we obtain Eq. (6). For completeness, it should be noted that we are making use of the assumption of negligible reverse reaction for (R2) as mentioned above. r2=k2[keto]rate=r2=k1k2k1[acetone[H3O+] Combining the individual step k 's into one overall k gives Eq. (7). rate=k[acetone][H3O+] The rate law stated in Eq. (7) is of the form described by Lapworth [2] in Eq. (2) above, such that the generally accepted three-step reaction mechanism stated in reactions (R1)(R3) is consistent with Lapworth's experimentally obtained rate law. Note that this does not depend on the reactant I2. Why is that
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


