Question: T = 20F T = 300F P2 = 60 psia 15 psia A mixture of ideal gases is contained in a closed, rigid container
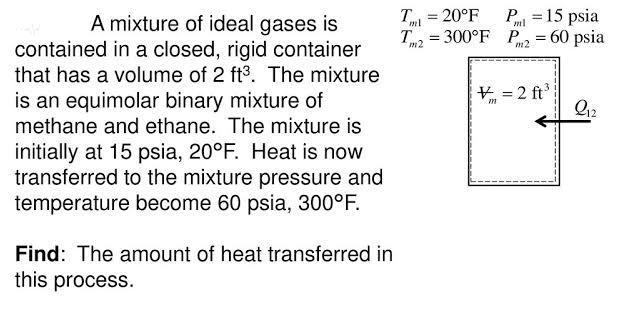
T = 20F T = 300F P2 = 60 psia 15 psia A mixture of ideal gases is contained in a closed, rigid container that has a volume of 2 ft3. The mixture %3! %3D ml ml m2 V = 2 ft' is an equimolar binary mixture of methane and ethane. The mixture is initially at 15 psia, 20F. Heat is now transferred to the mixture pressure and temperature become 60 psia, 300F. Find: The amount of heat transferred in this process.
Step by Step Solution
3.40 Rating (159 Votes )
There are 3 Steps involved in it
s Vm 2 Q12 Tm 20F 664C 266 40 K 664C 266 40 K 300F 42204 K ... View full answer

Get step-by-step solutions from verified subject matter experts


