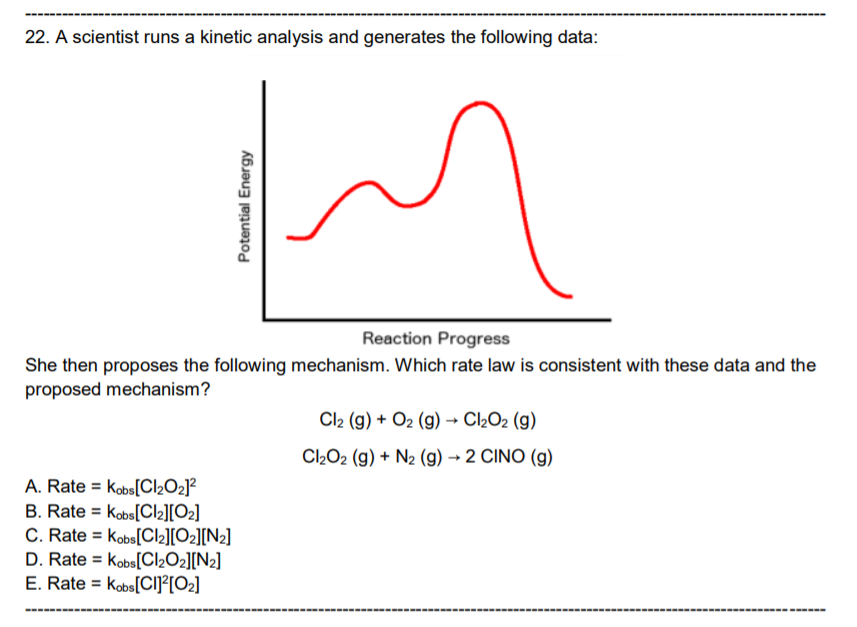
Question: Ans: C Please explain why the answer is C. Thank you. 22. A scientist runs a kinetic analysis and generates the following data: Potential Energy
Ans: C
Please explain why the answer is C. Thank you.
22. A scientist runs a kinetic analysis and generates the following data: Potential Energy Reaction Progress She then proposes the following mechanism. Which rate law is consistent with these data and the proposed mechanism? A. Rate = Kobs[ClO2] B. Rate = Kobs [Cl2][02] C. Rate = Kobs[Cl][O][N] D. Rate = Kobs [ClO][N] E. Rate = Kobs[CI][0] Cl (g) + O2 (g) ClO2 (g) ClO2 (g) + N (g) 2 CINO (g)
Step by Step Solution
3.41 Rating (164 Votes )
There are 3 Steps involved in it
activation energy is larger for 2nd step So 2nd step is rate determining step Fr... View full answer

Get step-by-step solutions from verified subject matter experts


