Question: Consider the combustion of methanol below. If 64 grams of methanol reacts with 160 grams of oxygen, what is the CHANGE in volume at
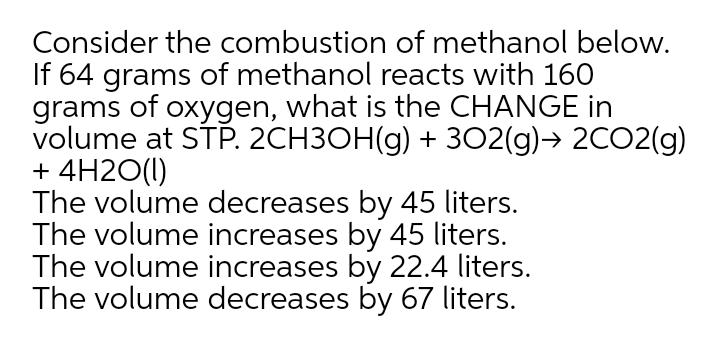
Consider the combustion of methanol below. If 64 grams of methanol reacts with 160 grams of oxygen, what is the CHANGE in volume at STP. 2CH3OH(g) + 3O2(g) 2CO2(g) + 4H2O(1) The volume decreases by 45 liters. The volume increases by 45 liters. The volume increases by 22.4 liters. The volume decreases by 67 liters.
Step by Step Solution
3.54 Rating (158 Votes )
There are 3 Steps involved in it
At STP mass of methanol taken 64 g mass of oxygen take... View full answer

Get step-by-step solutions from verified subject matter experts


