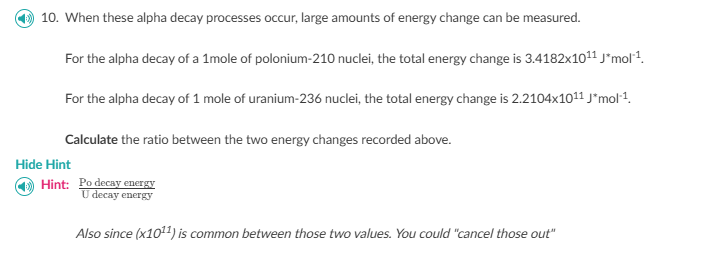
Question: answer (4) 10. When these alpha decay processes occur, large amounts of energy change can be measured. For the alpha decay of a Imole of
answer
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


