Question: answer 6 and 7 pls fast Using the Rydberg formula, calculate the initial energy level when an electron in a hydrogen atom transitions into n=3
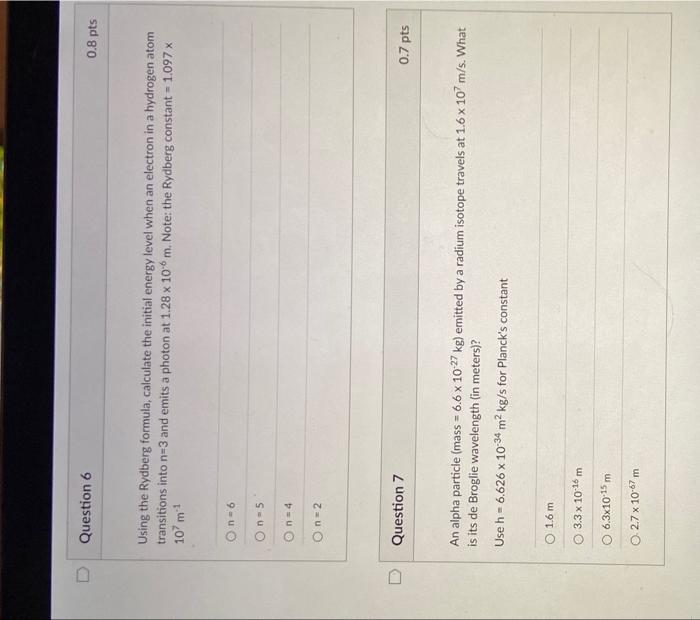
Using the Rydberg formula, calculate the initial energy level when an electron in a hydrogen atom transitions into n=3 and emits a photon at 1.28106m. Note: the Rydberg constant =1.097x 107m1 n=6n=5n=4n=2 Question 7 0.7pts An alpha particle (mass =6.61027kg ) emitted by a radium isotope travels at 1.6107m/s. What is its de Broglie wavelength (in meters)? Use h=6.6261034m2kg/s for Planck's constant 1.6m3.31016m6.31015m2.71067m
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


