Question: Answer ALL parts. Expressions ( some approximate ) for the 1 - D translational, rotational, and vibrational components of the molecular partition function for a
Answer ALL parts.
Expressions some approximate for the D translational, rotational, and vibrational
components of the molecular partition function for a diatomic molecule are given by
The symbols all take their usual meanings and the relevant energy levels are treated as
being those of the quantum mechanical square well, rigid rotor and simple harmonic oscillator respectively.
a For a typical diatomic molecule at room temperature, state the order, from smallest to largest of and Explain your reasoning for giving your answer in terms of the expected energy levels and typical quantum numbers populated in each case.
b Explain how the D partition function for translation, is used to make the partition function for translation in a D cuboid container. Your answer should include the resultant form of the translational partition function in D
Question continued.
c Starting with the expression for given above, show that the average energy stored in rotational motion of a diatomic molecule increases linearly with temperature.
d Explain the result from part c in terms of the principle of equipartition and the quantum mechanical behaviour of a rigid rotor.
quantum mechanical behaviour of a rigid rotor.
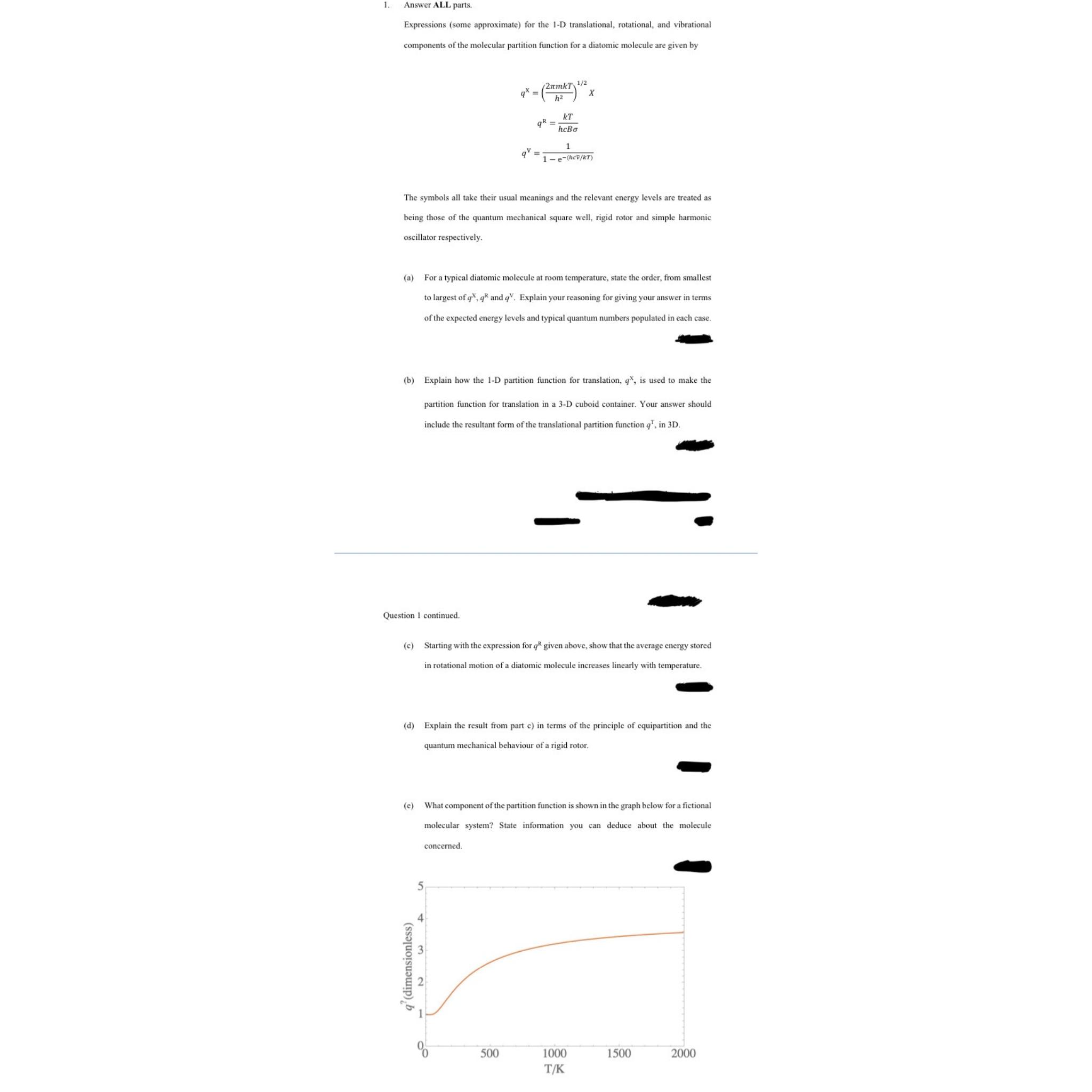
e What component of the partition function is shown in the graph below for a fictional molecular system? State information you can deduce about the molecule concerned.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


