Question: answer all parts with explaination, will give thumbs up ! :) A mixture containing 21% by weight of phenol in water and weighing 135 grams
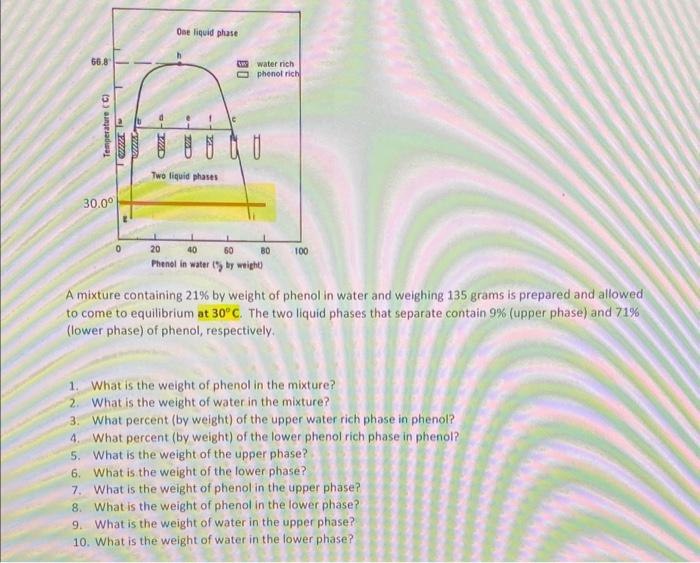
A mixture containing 21% by weight of phenol in water and weighing 135 grams is prepared and allowed to come to equilibrium at 30C. The two liquid phases that separate contain 9% (upper phase) and 71% (lower phase) of phenol, respectively. 1. What is the weight of phenol in the mixture? 2. What is the weight of water in the mixture? 3. What percent (by weight) of the upper water rich phase in phenol? 4. What percent (by weight) of the lower phenol rich phase in phenol? 5. What is the weight of the upper phase? 6. What is the weight of the lower phase? 7. What is the weight of phenol in the upper phase? 8. What is the weight of phenol in the lower phase? 9. What is the weight of water in the upper phase? 10. What is the weight of water in the lower phase
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


