Question: Answer all questions 4-8. 5. Combining what you know from 1-5 above, draw the structure of your compound ( 4 pts) indicating with subscripts matching
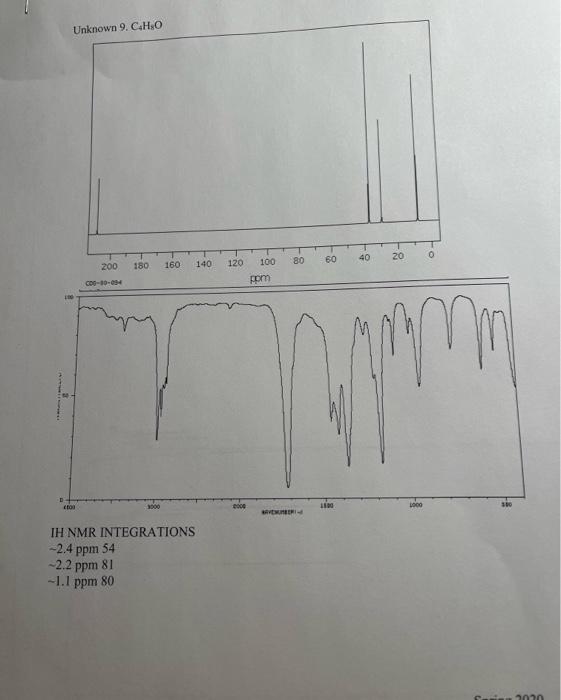
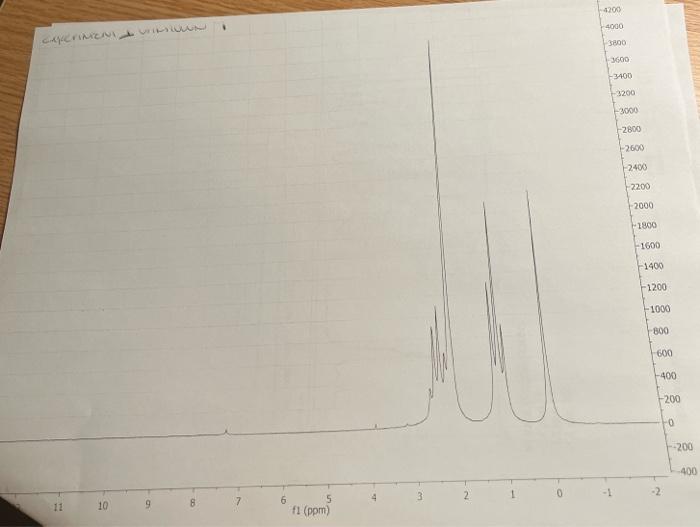
5. Combining what you know from 1-5 above, draw the structure of your compound ( 4 pts) indicating with subscripts matching the letters used on the table (1pt), the identity of each hydrogen. For exampli 6. How many distinct carbons are present in your 13C NMR? Each 'line' is a separate carbon. List the shift of each carbon from your 13C in the appropriate category below. (Read the shift of each 'line' in the 13C, place that number in the correct category.) (2 pt) Carbonyl= Aromatic\&/or Alkene = Alkyne = CO= Alkane= 7. If the number of distinct carbons in the 13C NMR are less than the total number of carbons in your empirical formula, explain using your structure. It may help to draw your structure and indicate the different and identical carbons. Be clear. ( 2pt ) If the number of distinct carbons, equals the total in your formula, then explain why isopropyl benzene (C9H12) only shows 6 unique carbons. 8. Label each carbon of proposed structure with a possible 13C shift from your 13C spectrum. (Match each carbon line in the 13C, to a particular carbon in your structure). Be specific. (1 pts) IH NMR INTEGRATIONS 2.4ppm54 2.2ppm81 1.1 ppm 80 ceperimicer U unsivens
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


