Question: Answer all questions completely(Table 1, 2, 3): Page 1 > of 19 ZOOM Experiment1 Chemical Reactions and Net Lonic Equations I. Objective: To predict the
Answer all questions completely(Table 1, 2, 3):


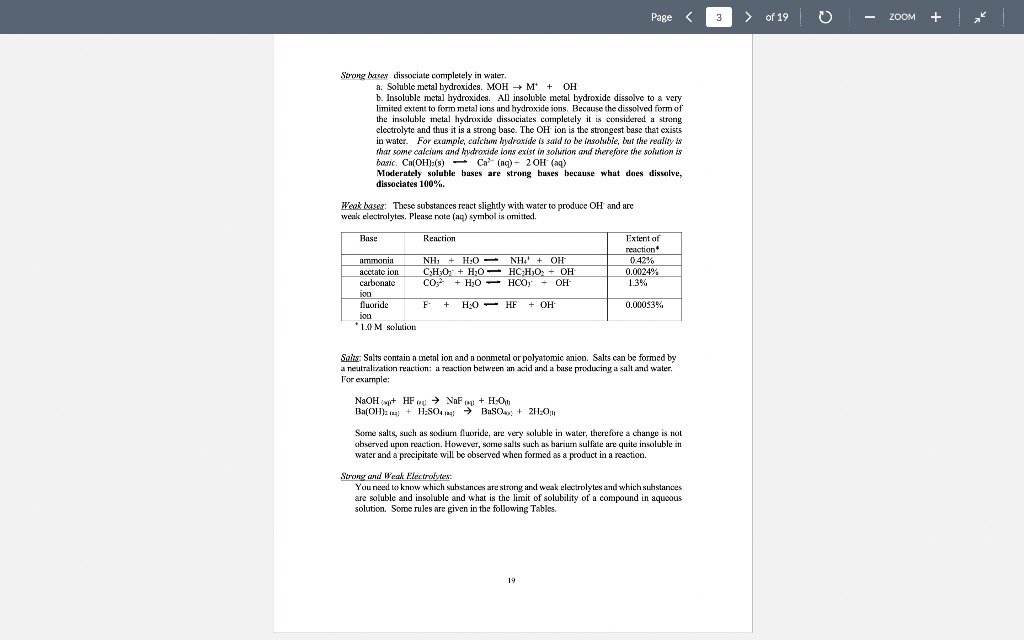
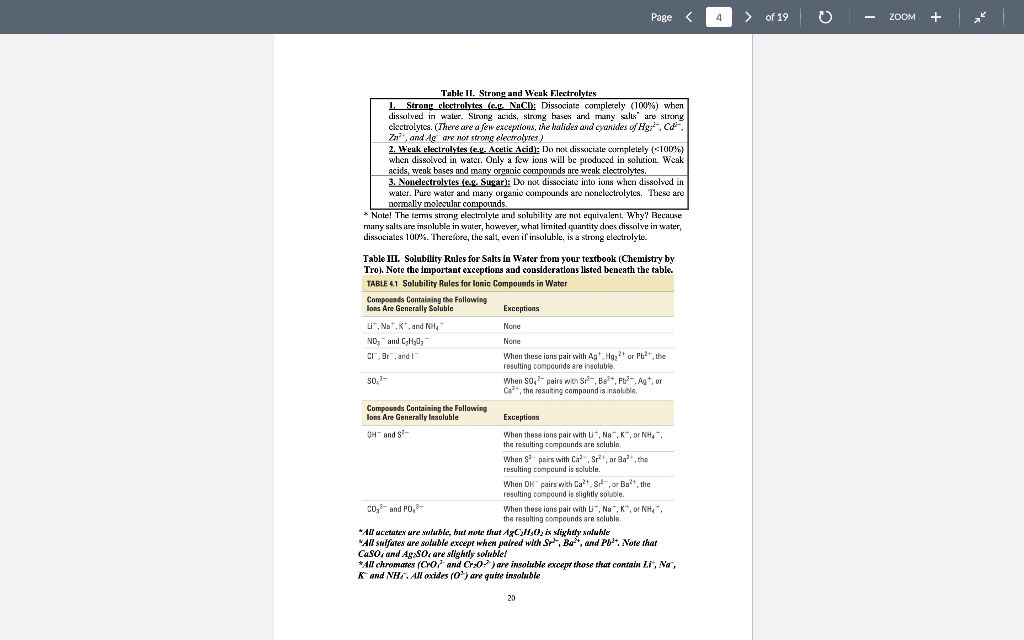


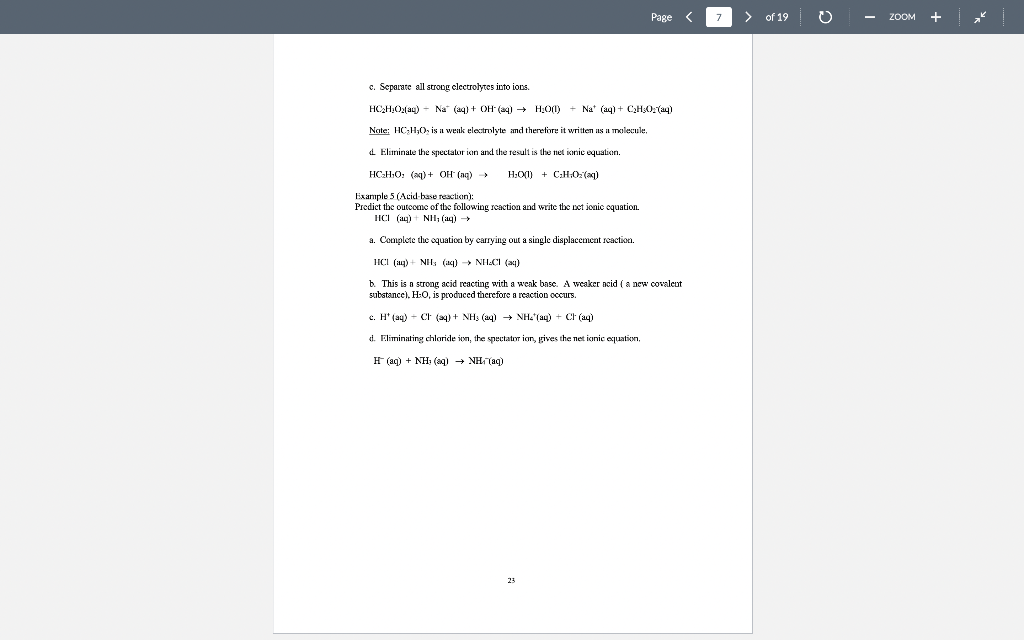
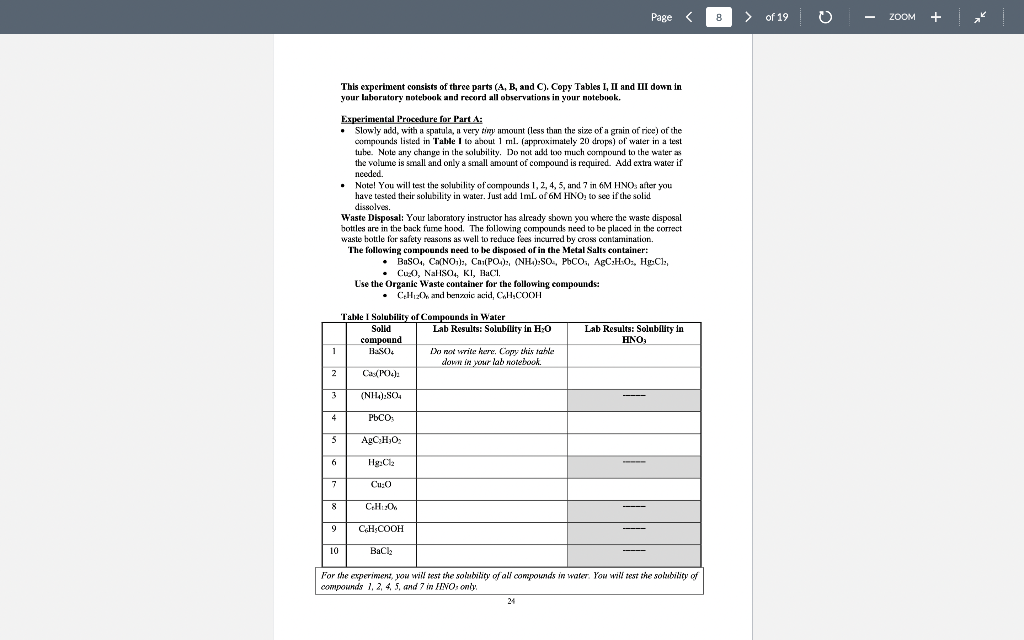

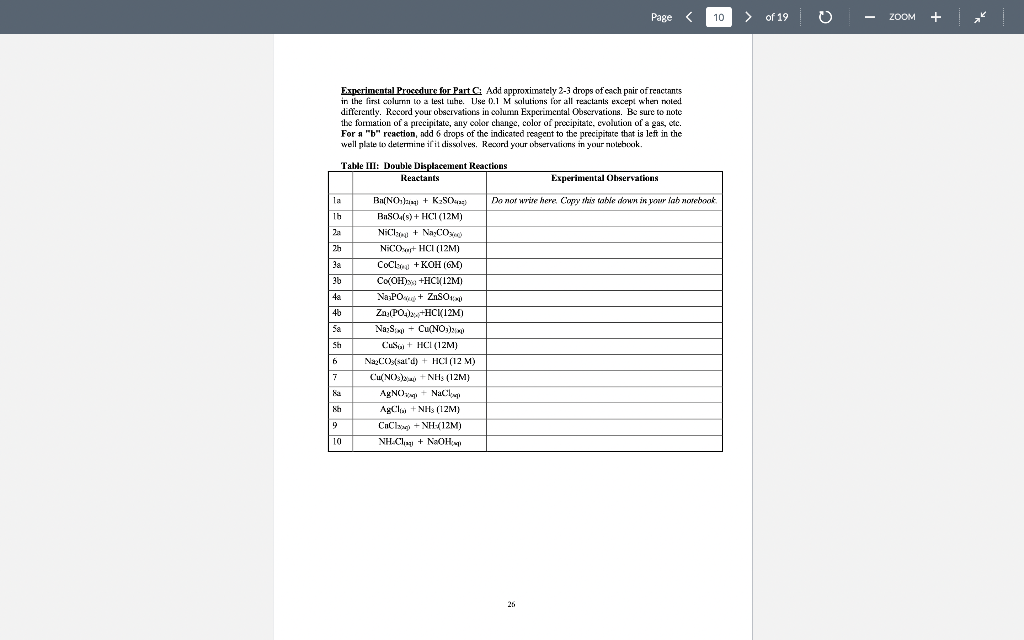
Page 1 > of 19 ZOOM Experiment1 Chemical Reactions and Net Lonic Equations I. Objective: To predict the products of some displacement reactions and write net ionic equations Note-the(n) has been eliminated from all equations below while (s) () and (c) have been used to represent phases. When you write chemical reactions in your Jah report you show all phases including lug). II. Chemical Principles A. Reaction Types 1. Combination. This reaction tyre inclves the combining of elements d'or pounds to form a new element 2 Mg(s) + + element > Oxls) compound 2 Mgois) 2. Descomposition. This ration type involves ile splitting part of a single compound to me compounds ar els > compound new compound and/or elements 2 KCTO () 2 KCl) + 3029) 3. Single Replacement. This reaction type involves an element displacing another element orion in a compound, or a chemical compound displacing an element or ion from another compound + clements + compound clement, + compound , + Zn(s) 2 HICI (1) H:(1) ZnCU) 4. Double Displacement (Menthesis). This reaction type involves the exchange of clemcuts or ions in two compounds Double displacement reactions will be the focus of this experiment. compound,+ compound compounde + conupounds a. precipitation: AgNO: (84) + NaBe (aq) AgBr(s) + NaNO, (aq) b. dissolut CuO(s) + 2 HCI (0) > H:0(1) + CuCl(aq) 17 Page 2 > of 19 ZOOM c. neutralizatim HNO, (1) ng + NaOH (ng) NaNO, (aq) +HO () gus firming CUSS) + 2HCI HS(aq) + CuCl(aq) c. Complex io formation (not technically a double-displacement reaction) Transition melalls are your electrcin acceptints and are capable of complexing while neutral molecules (and some anions) to form a new complex ior. Complexions reactions are common in qualitative annlysis. An example is shown below. AgCl, + 2N = /AgNII, 41* 127+ Clima + 1 - To be able to predict the products of these renctions, you must know the identity nod priperties of iconic compounds, acicls arxl bases, and molecular species. B. Predicting Products of Displacement Reactions and Writing Net Ionic Equations. Strong delas. All strong acids completely dissociate in water, for example: TICIO4, HC1, 1r, HII, TINC., 11,804 a. bitric acid HNO: + HAO() + H2O + NO3 h, sulfuric acid: H-SO. : HO) + HSC HSO4 + HOD) H0 + SO4 + ( HY + The first ionization step for sulfuric acid is 100%, bowever the second step is an equilibrium situation and iconization is only partial Note: Molecules of strong acids such as HCO and H_SO4 do not exist in slution because we real them as ionizing 100% that is as strong electrolyles. Haz) can be writtca in place of H:0, the hydronium ion. Menze Acide These acids renct only slightly with water to produce the hydronium ion. They, however, will react completely with a strong base. Please note (24) symbol is omitted. Acid Reaction Extent ol Rcaction 0.42% cetic acid carbonic acid 0.4%65% HKCH10, 11:00) II.CO), HO(1) TICO, , + CD TOXU) NIE! 11:0(1) - 111 HO + CH10 110) + HICO), TO CO2- 110) NII + : D.XI24% Kinium KM * 1.0M solution 1K Page 3 > of 19 ZOOM Soong his dissociate completely in water a. Soluble metal bydroxides. MOH M + OH b. Insoluble metal hydroxidcs. All insoluble metal Lydroxide dissolve to a very limited extent to form metal ions and hydroxide ions. Because the dissolved form of the insoluble metal hydroxide dissociates completely it is considered a strong electrolyte and thus it is a strong base. The OH ion is the strongest base that exists in water. For example, calcium hydroxide is said to be insoluble, bur the reality is that some calcium and hydroxide ions exist in solution and therefore the solution is basic Ca(OH);(8) Car (ng) - 20H (aq) . ) - Moderately soluble buses are strong buses because what does dissolve, dissociates 100% Wenkkaus: These substances react slightly with water to produce OH and are werk electrolytes. Please rate (aa) symbol is omittel. Base Reaction NH, + H30 - NH4+ + OH ' + CH:0,- + HO HO HO + OH ; CO; + H20 - HOO," + OH Extent of reaction 0.42% 0.0024% 1.3% Immanin acetate ion carbonate 100 fluoride iou * 1.0M solution F + + HO H:0 HF + OH 0.00053% Saits: Salts contain a metal ion and a Donmetal or polyatomic anion. Salts can be formed by a neutralization reaction: il reaction between an acid anxi a bese producing a salt and water. For example: NaOH + HF ou NaF + H-01 Ba(OH). 13 + H.SO BuSO + 2H1:0,11 Some salls, such as sodiurn fluoride, are very soluble in water, therefore a change is not observed upon reaction. However, some salts such as bariurn sulfate are quite insoluble in water and a precipitate will be observed when formed as a product in a reaction. Soring and Weat Electrolytes You need to know which substances are strong and weak electrolytes and which substances are soluble and insoluble and what is the limit of solubility of a compound in aqueous solution. Some rules are given in the following Tables 19 Page 4 4 > of 19 0 ZOOM Table II. Strong and Weak Electrolytes 1. Strong clectrolytes (c. NACI: Dissociate completely (100%) when dissolved in water. Strong acus, strumg hases and many sales are strong clectrolytes. (There are a few excepxions, the kalides and candles oHgzt, Znetand Ag are not stwig elevwales) 2. Weuk electrolytes (ey. Acetic Acid): Do nx dissicisle oxunpletely (+100%) when dissolved in water. Only a few ions will be produced in solution. Weak acids, weak bases and many organic compounds are weak electrolytes. 3. Nonelectrolytes (ec. Suvar): Do not dissociate into ions when dissolved in water. Pure water and many organic compounds are moneloctrolytes. These are pompally molecular compounds. * Note! The tems streng electrolyte and solubility are not equivalent. Why? Because miny salts are insoluble in winter, however, what limited quantity cloes dissolve in water, dissociates 100%. Therefore, the salt, even if insoluble, is a strong electrolyte Table III. Solubility Rules for Salts in Water from your textbook (Chemistry by Tro). Note the important exceptions and considerations listed beneath the table. TABLE 41 Solubility Rules for lonic Compounds in Water Compounds Containing the following lons Are Generally Soluble Exceptions Lit. Nat. K, and NHA None ND, and C H20,- None CI,Br", and When these ions pair with Aat. Hg2+ or put the resulting compounds are insoluble 50,- When 50,- pairs with Sp. Bo P., or Co the resulting compound is insoluble Compounds Containing the following lons Are Generally Insoluble Exceptions OH and S? When these ions pair with U*, Na, K, or NH4 the resulting compounds are soluble When pairs with Ca, Sr?, or the resulting compound is soluble. When DH"pairs with Cat Spor Bat the resulting compound is slightly soluble co, and POS- When these ions pair with U", No, k*, or NH4 the resulting compounds are soluble * Allucetatex ure solublc, huet note ut AgC-3130, is slightly swuhle *All sulfates are soluble except when paired with Sr, But, and Pb. Note that CaSO and Ag SO, are slightly soluble! *All chromates (Cror and Cr:0") are insoluble except those that contain Li, Na, K and NH, . All oxides (O") are quite insoluble 20 Page 5 > of 19 o ZOOM Solubility is defined as the maximum amount of substance that dissolves in a given amount of solvent al a giveni lemperature. The solubility limit ofinany compounds can be found in the Handbook of Chemistry and Physics. C. Rules for Writing Net Tonic Equations 1. While the overall Ixalanced rolecular" equaticin. 2. Rewrite the molecular equation so that only soluble, strong cloctrolytes are separated into their ions 3. Eliminate spccics common to the reactants and products (spectatorious). 4. The resultant equation is the bet ionic equation 5. There is no net ionic equatim if there is no citiem D. Application of Net Ionie Equation Rules. Example 1: (Iruxluclin ola wilid) Predict the outcome of the following reaction and write the net ionic equation, MgCl(aq) + K-CO (aq) ? a. Complete the equatic by carrying ou a double replacement reaction (exchange partners MgCl; (24) + K 00 (24) 2 KCl (24) MgCO:(?) b. Use rules 1.A -6.4, 1.B -3.B, and I.C-7.C to determine if a rcaction occured Accounting to 6.C, MgCO; is insoluble and will precipitate, write a (s) next to the compound. According to 1A, il reaction xxlir c. Write the molecular equation and then apply rules given above lo separate strong clectrolytes into their ion forms and identify magnesium carbonate as an insoluble My (4) K 00, (24) 2 KCI (24) + Myxo(s) My() CH4) 2K () CX), (14) 2K (14) 2CH(14) MyCO) d. Eliminate ions common to reactants and products and the result is the net ionic equation: My? (I) + CC) (14) MyCO.(8) 21 Page 6 6 > of 19 0 ZOOM Example 2: (Production of Gas) Predict the outcome of the reaction of hydrochloric acid with M&COXS). a. Complete the double displacement reaction MgCO:(8) +2HCI (aq) + MgCl (aq) + H2CO3(a) M MgCh (aq) - HoH+ - HOXH) COD Note: H-C0. is a weak acid that in the presence of heat decomposes to H:O(l) and COAR), We nomally show carbonic acid in the decomposed form. b. Use the rules to determine if a reaction has occurred. A reaction occurs because carbonic acid forms and it is a weak acid and weak electrolyte. c. Separate the soluble and strong electrolytes into ions. MgCO-(8) + 2H (n) - 201 (aq) + Mg (aq) + 2Cl(aq) + H:01) - Co-g) d. Eliminate the common sens and the result is the net iconic equation, MgCO:(8) +2H Mg(aq) + H:01) + CO_g) Example 3: (No observer reaction) Predict the result of the following reaction and write the net iconic equalin. NaCl (x) + Cu(NO3)2 (aq) ? a. Complete the equation by carrying out a double clisplacement reaction, 2 NaCl (aq) + Ca(NO:) (aq) Calxaq) + 2 NaNO3(ng) b. Use the rules to determine if a reaction has occurred. Both CuCI: (S.C) and NaNO: (2.C) are soluble and strong electrolytes. Thus no reaction can occur as the requirements of Rules 1.A - S. A are not met. c. 2Na + 2C + Cu+ + 2NO; Cult + 2Cl + 2Nnt + 2NO, d. All the ions cancel and thus there is no net ionic equation. Example 4: (Acid-base reaction) Predict the outcome of the following reaction and write the net ionic equation. HC H0 (aq) + NaOH (aq) + a. Complete the bilanced equation by carrying out a double displacement reaction, HCH0 (aq) + Na 011 (4) + H:O() Na Ha + b. Has a reaction occurred? Water is a new covalent substance (2.A), so a reaction bas occurred. 22 Page 7 > of 19 - ZOOM c. Separate all strong electrolytes into ions. HC.HOLL) - Na (aq) + OH- (aq) HOM) + Nat (aq) + CH:0 (ay) Note: HC-H30, is il wenk electrolyte and therefore it written ins il molecule, Eliminate the spectatorian and the result is the net inic equation. HC.H.0: (M) + OH(aq) H:O(1) + C:H:02 (1) Example 5 (Acid base reaction: Predict the outcome of the following reaction and write the actionic equation. HCI (14) NII(14) a. Complete the equation by carrying out a single displacement reactice. HCI (14)+NHS () NILCI (4) b. This is a strong acid reacting with a weak base. A weaker acid ( a new covaleut substance). H:0, is produced therefore a reaction occurs. c. H (8 } - { {4} + NH3 (a + NH auj + Cl (au) d. Eliminating chloride in the spectator ion, gives the neticnic ecuation, H(aq) + NH: (aq) + NH(aq) Page 8 8 > of 19 ZOOM This experiment consists of three parts (A, B, and C). Copy Tables I, II and III down in your luborutory notebook and record all observations in your notebook. Esperimental Procedure for Part : Slowly add, with a spatula, a very tiny amount (less than the size of a grain of rice) of the compounds listed in Table I to about 1 ml. (approximately 20 drops) of water in a test tube. Note any change in the solubility. Do not aukt to much compound to the water as the volume is small and only a small amount of compound is required. Add extra water if needed. Note! You will test the solubility of compounds 1, 2, 4, 5, and 7 in 6M HNO, after you have tested their solubility in water. Just add ImL of 6M HNO, to see if the solid dissolves Waste Disposal: Your laboratory instructor has already show you where the waste disposal bottles are in the back tume hood. The following compounds need to be placed in the correct waste hottle for safety reasons as well to reduce fees incurred by cross contamination. The following compounds need to be disposed of in the Metal Salts container: BuSO4, Ca(NO), C (PO4): (NH):SO, POCO: AgC:H:0, HCl, Cup, Nal ISO4, KI, HCl. Use the Organic Waste container for the following compounds: CHI and henzeic acid, C.H.COOH Lab Results: Solubility in HNO, Talvle I Solubility of Compounds in Water Solid Lab Results: Solubility in H:0 compound HS) I write herr. Copy this tahe down in your lab wotebook CIN):): 1 1 2 3 (NHL):80, 4 PbCO 5 AgC H,O: 6 Hg.Ch 7 Cu: 8 CHO 9 CH3COOH 10 BaCE For the experiment, you will test the solubility of all compounds in water. You wil test the solubility of compounds /. 2, 4, 5, and 7 in LINO, only Page of 19 - ZOOM Experimental Procedure for Part B: Place cach compound in the first column in a well of a spot plate. If a compound is a solid and next aliquid, add a small amount of water to it and stir. For all solutions, use 0.1 M provided in lah. Use a conductivity device to determine if the substance is conducting or not. Record the relative brightness of the light ar absence of light. A bright ligbt only indicates morc ions are in solution compared to a dim light, not whether it is a strong or werk electrolyte. The concentration of ions must he known to make a more definitive conclusion. The absence of a light indicates that ions are not present. Only the ends of the two oupper pubes of the device should be in the solution. On the back of the conductivity device is a guide for you to use E Tahle II: Electrolytes Lab Results from Conductivity device Compound HNO: 1 Do not write here. Copy this izhle down yowlab notebook HCHI 3 NH NaOH 5 CH(OH). 6 K CO, Cu(NO) 8 Nici: 9 Zn(NO3)2 10 CH OH (othanol) Page 10 > of 19 ZOOM Experimental Procedure for Part : Add approximately 2-3 drops of each pair of reactants in the first column lo a lesi Luhe. Use 0.1 M solutions for all reacliants except when nxitext differently. Record your observations in column Experimental Observations. Be sure to note the formation of a precipitatc, any color change, color of precipitate, evolution of a gas, ate. For a "b" reaction, add 6 drops of the indicated reagent to the precipitate that is left in the well plate to determine if it dissolves. Recxind your observations in your notebook. Table IT: Double Displacement Reactions Reactants Experimental Observations Da nor wire here Coyyy this table down in your lazo narebook la 1b za Bi(NO) + K2SO4) BaSO4(s)+ HCI(12M) ) NiCl + NaCO NiCor+ HCI (12M) 2 38 21 30 de 4a 46 5. Sa Sh CoC +KOH (GM) COH+HCHI2M COOH) THCHIZMI ) + Na PO + ZnS LIST Zn:(PO4)2, +HCK12M) x NASCUNO3)2 + S + H2H4 () Na2COA sald) + HCI(12 M) )2 + NH3 (12M) AgNOX + NaCl AgCl + NH3 (12M) CnChai + NH412M) NH.CI + NaOH 6 7 CNC + NH3 (12) Nh 2 9 10 Page 1 > of 19 ZOOM Experiment1 Chemical Reactions and Net Lonic Equations I. Objective: To predict the products of some displacement reactions and write net ionic equations Note-the(n) has been eliminated from all equations below while (s) () and (c) have been used to represent phases. When you write chemical reactions in your Jah report you show all phases including lug). II. Chemical Principles A. Reaction Types 1. Combination. This reaction tyre inclves the combining of elements d'or pounds to form a new element 2 Mg(s) + + element > Oxls) compound 2 Mgois) 2. Descomposition. This ration type involves ile splitting part of a single compound to me compounds ar els > compound new compound and/or elements 2 KCTO () 2 KCl) + 3029) 3. Single Replacement. This reaction type involves an element displacing another element orion in a compound, or a chemical compound displacing an element or ion from another compound + clements + compound clement, + compound , + Zn(s) 2 HICI (1) H:(1) ZnCU) 4. Double Displacement (Menthesis). This reaction type involves the exchange of clemcuts or ions in two compounds Double displacement reactions will be the focus of this experiment. compound,+ compound compounde + conupounds a. precipitation: AgNO: (84) + NaBe (aq) AgBr(s) + NaNO, (aq) b. dissolut CuO(s) + 2 HCI (0) > H:0(1) + CuCl(aq) 17 Page 2 > of 19 ZOOM c. neutralizatim HNO, (1) ng + NaOH (ng) NaNO, (aq) +HO () gus firming CUSS) + 2HCI HS(aq) + CuCl(aq) c. Complex io formation (not technically a double-displacement reaction) Transition melalls are your electrcin acceptints and are capable of complexing while neutral molecules (and some anions) to form a new complex ior. Complexions reactions are common in qualitative annlysis. An example is shown below. AgCl, + 2N = /AgNII, 41* 127+ Clima + 1 - To be able to predict the products of these renctions, you must know the identity nod priperties of iconic compounds, acicls arxl bases, and molecular species. B. Predicting Products of Displacement Reactions and Writing Net Ionic Equations. Strong delas. All strong acids completely dissociate in water, for example: TICIO4, HC1, 1r, HII, TINC., 11,804 a. bitric acid HNO: + HAO() + H2O + NO3 h, sulfuric acid: H-SO. : HO) + HSC HSO4 + HOD) H0 + SO4 + ( HY + The first ionization step for sulfuric acid is 100%, bowever the second step is an equilibrium situation and iconization is only partial Note: Molecules of strong acids such as HCO and H_SO4 do not exist in slution because we real them as ionizing 100% that is as strong electrolyles. Haz) can be writtca in place of H:0, the hydronium ion. Menze Acide These acids renct only slightly with water to produce the hydronium ion. They, however, will react completely with a strong base. Please note (24) symbol is omitted. Acid Reaction Extent ol Rcaction 0.42% cetic acid carbonic acid 0.4%65% HKCH10, 11:00) II.CO), HO(1) TICO, , + CD TOXU) NIE! 11:0(1) - 111 HO + CH10 110) + HICO), TO CO2- 110) NII + : D.XI24% Kinium KM * 1.0M solution 1K Page 3 > of 19 ZOOM Soong his dissociate completely in water a. Soluble metal bydroxides. MOH M + OH b. Insoluble metal hydroxidcs. All insoluble metal Lydroxide dissolve to a very limited extent to form metal ions and hydroxide ions. Because the dissolved form of the insoluble metal hydroxide dissociates completely it is considered a strong electrolyte and thus it is a strong base. The OH ion is the strongest base that exists in water. For example, calcium hydroxide is said to be insoluble, bur the reality is that some calcium and hydroxide ions exist in solution and therefore the solution is basic Ca(OH);(8) Car (ng) - 20H (aq) . ) - Moderately soluble buses are strong buses because what does dissolve, dissociates 100% Wenkkaus: These substances react slightly with water to produce OH and are werk electrolytes. Please rate (aa) symbol is omittel. Base Reaction NH, + H30 - NH4+ + OH ' + CH:0,- + HO HO HO + OH ; CO; + H20 - HOO," + OH Extent of reaction 0.42% 0.0024% 1.3% Immanin acetate ion carbonate 100 fluoride iou * 1.0M solution F + + HO H:0 HF + OH 0.00053% Saits: Salts contain a metal ion and a Donmetal or polyatomic anion. Salts can be formed by a neutralization reaction: il reaction between an acid anxi a bese producing a salt and water. For example: NaOH + HF ou NaF + H-01 Ba(OH). 13 + H.SO BuSO + 2H1:0,11 Some salls, such as sodiurn fluoride, are very soluble in water, therefore a change is not observed upon reaction. However, some salts such as bariurn sulfate are quite insoluble in water and a precipitate will be observed when formed as a product in a reaction. Soring and Weat Electrolytes You need to know which substances are strong and weak electrolytes and which substances are soluble and insoluble and what is the limit of solubility of a compound in aqueous solution. Some rules are given in the following Tables 19 Page 4 4 > of 19 0 ZOOM Table II. Strong and Weak Electrolytes 1. Strong clectrolytes (c. NACI: Dissociate completely (100%) when dissolved in water. Strong acus, strumg hases and many sales are strong clectrolytes. (There are a few excepxions, the kalides and candles oHgzt, Znetand Ag are not stwig elevwales) 2. Weuk electrolytes (ey. Acetic Acid): Do nx dissicisle oxunpletely (+100%) when dissolved in water. Only a few ions will be produced in solution. Weak acids, weak bases and many organic compounds are weak electrolytes. 3. Nonelectrolytes (ec. Suvar): Do not dissociate into ions when dissolved in water. Pure water and many organic compounds are moneloctrolytes. These are pompally molecular compounds. * Note! The tems streng electrolyte and solubility are not equivalent. Why? Because miny salts are insoluble in winter, however, what limited quantity cloes dissolve in water, dissociates 100%. Therefore, the salt, even if insoluble, is a strong electrolyte Table III. Solubility Rules for Salts in Water from your textbook (Chemistry by Tro). Note the important exceptions and considerations listed beneath the table. TABLE 41 Solubility Rules for lonic Compounds in Water Compounds Containing the following lons Are Generally Soluble Exceptions Lit. Nat. K, and NHA None ND, and C H20,- None CI,Br", and When these ions pair with Aat. Hg2+ or put the resulting compounds are insoluble 50,- When 50,- pairs with Sp. Bo P., or Co the resulting compound is insoluble Compounds Containing the following lons Are Generally Insoluble Exceptions OH and S? When these ions pair with U*, Na, K, or NH4 the resulting compounds are soluble When pairs with Ca, Sr?, or the resulting compound is soluble. When DH"pairs with Cat Spor Bat the resulting compound is slightly soluble co, and POS- When these ions pair with U", No, k*, or NH4 the resulting compounds are soluble * Allucetatex ure solublc, huet note ut AgC-3130, is slightly swuhle *All sulfates are soluble except when paired with Sr, But, and Pb. Note that CaSO and Ag SO, are slightly soluble! *All chromates (Cror and Cr:0") are insoluble except those that contain Li, Na, K and NH, . All oxides (O") are quite insoluble 20 Page 5 > of 19 o ZOOM Solubility is defined as the maximum amount of substance that dissolves in a given amount of solvent al a giveni lemperature. The solubility limit ofinany compounds can be found in the Handbook of Chemistry and Physics. C. Rules for Writing Net Tonic Equations 1. While the overall Ixalanced rolecular" equaticin. 2. Rewrite the molecular equation so that only soluble, strong cloctrolytes are separated into their ions 3. Eliminate spccics common to the reactants and products (spectatorious). 4. The resultant equation is the bet ionic equation 5. There is no net ionic equatim if there is no citiem D. Application of Net Ionie Equation Rules. Example 1: (Iruxluclin ola wilid) Predict the outcome of the following reaction and write the net ionic equation, MgCl(aq) + K-CO (aq) ? a. Complete the equatic by carrying ou a double replacement reaction (exchange partners MgCl; (24) + K 00 (24) 2 KCl (24) MgCO:(?) b. Use rules 1.A -6.4, 1.B -3.B, and I.C-7.C to determine if a rcaction occured Accounting to 6.C, MgCO; is insoluble and will precipitate, write a (s) next to the compound. According to 1A, il reaction xxlir c. Write the molecular equation and then apply rules given above lo separate strong clectrolytes into their ion forms and identify magnesium carbonate as an insoluble My (4) K 00, (24) 2 KCI (24) + Myxo(s) My() CH4) 2K () CX), (14) 2K (14) 2CH(14) MyCO) d. Eliminate ions common to reactants and products and the result is the net ionic equation: My? (I) + CC) (14) MyCO.(8) 21 Page 6 6 > of 19 0 ZOOM Example 2: (Production of Gas) Predict the outcome of the reaction of hydrochloric acid with M&COXS). a. Complete the double displacement reaction MgCO:(8) +2HCI (aq) + MgCl (aq) + H2CO3(a) M MgCh (aq) - HoH+ - HOXH) COD Note: H-C0. is a weak acid that in the presence of heat decomposes to H:O(l) and COAR), We nomally show carbonic acid in the decomposed form. b. Use the rules to determine if a reaction has occurred. A reaction occurs because carbonic acid forms and it is a weak acid and weak electrolyte. c. Separate the soluble and strong electrolytes into ions. MgCO-(8) + 2H (n) - 201 (aq) + Mg (aq) + 2Cl(aq) + H:01) - Co-g) d. Eliminate the common sens and the result is the net iconic equation, MgCO:(8) +2H Mg(aq) + H:01) + CO_g) Example 3: (No observer reaction) Predict the result of the following reaction and write the net iconic equalin. NaCl (x) + Cu(NO3)2 (aq) ? a. Complete the equation by carrying out a double clisplacement reaction, 2 NaCl (aq) + Ca(NO:) (aq) Calxaq) + 2 NaNO3(ng) b. Use the rules to determine if a reaction has occurred. Both CuCI: (S.C) and NaNO: (2.C) are soluble and strong electrolytes. Thus no reaction can occur as the requirements of Rules 1.A - S. A are not met. c. 2Na + 2C + Cu+ + 2NO; Cult + 2Cl + 2Nnt + 2NO, d. All the ions cancel and thus there is no net ionic equation. Example 4: (Acid-base reaction) Predict the outcome of the following reaction and write the net ionic equation. HC H0 (aq) + NaOH (aq) + a. Complete the bilanced equation by carrying out a double displacement reaction, HCH0 (aq) + Na 011 (4) + H:O() Na Ha + b. Has a reaction occurred? Water is a new covalent substance (2.A), so a reaction bas occurred. 22 Page 7 > of 19 - ZOOM c. Separate all strong electrolytes into ions. HC.HOLL) - Na (aq) + OH- (aq) HOM) + Nat (aq) + CH:0 (ay) Note: HC-H30, is il wenk electrolyte and therefore it written ins il molecule, Eliminate the spectatorian and the result is the net inic equation. HC.H.0: (M) + OH(aq) H:O(1) + C:H:02 (1) Example 5 (Acid base reaction: Predict the outcome of the following reaction and write the actionic equation. HCI (14) NII(14) a. Complete the equation by carrying out a single displacement reactice. HCI (14)+NHS () NILCI (4) b. This is a strong acid reacting with a weak base. A weaker acid ( a new covaleut substance). H:0, is produced therefore a reaction occurs. c. H (8 } - { {4} + NH3 (a + NH auj + Cl (au) d. Eliminating chloride in the spectator ion, gives the neticnic ecuation, H(aq) + NH: (aq) + NH(aq) Page 8 8 > of 19 ZOOM This experiment consists of three parts (A, B, and C). Copy Tables I, II and III down in your luborutory notebook and record all observations in your notebook. Esperimental Procedure for Part : Slowly add, with a spatula, a very tiny amount (less than the size of a grain of rice) of the compounds listed in Table I to about 1 ml. (approximately 20 drops) of water in a test tube. Note any change in the solubility. Do not aukt to much compound to the water as the volume is small and only a small amount of compound is required. Add extra water if needed. Note! You will test the solubility of compounds 1, 2, 4, 5, and 7 in 6M HNO, after you have tested their solubility in water. Just add ImL of 6M HNO, to see if the solid dissolves Waste Disposal: Your laboratory instructor has already show you where the waste disposal bottles are in the back tume hood. The following compounds need to be placed in the correct waste hottle for safety reasons as well to reduce fees incurred by cross contamination. The following compounds need to be disposed of in the Metal Salts container: BuSO4, Ca(NO), C (PO4): (NH):SO, POCO: AgC:H:0, HCl, Cup, Nal ISO4, KI, HCl. Use the Organic Waste container for the following compounds: CHI and henzeic acid, C.H.COOH Lab Results: Solubility in HNO, Talvle I Solubility of Compounds in Water Solid Lab Results: Solubility in H:0 compound HS) I write herr. Copy this tahe down in your lab wotebook CIN):): 1 1 2 3 (NHL):80, 4 PbCO 5 AgC H,O: 6 Hg.Ch 7 Cu: 8 CHO 9 CH3COOH 10 BaCE For the experiment, you will test the solubility of all compounds in water. You wil test the solubility of compounds /. 2, 4, 5, and 7 in LINO, only Page of 19 - ZOOM Experimental Procedure for Part B: Place cach compound in the first column in a well of a spot plate. If a compound is a solid and next aliquid, add a small amount of water to it and stir. For all solutions, use 0.1 M provided in lah. Use a conductivity device to determine if the substance is conducting or not. Record the relative brightness of the light ar absence of light. A bright ligbt only indicates morc ions are in solution compared to a dim light, not whether it is a strong or werk electrolyte. The concentration of ions must he known to make a more definitive conclusion. The absence of a light indicates that ions are not present. Only the ends of the two oupper pubes of the device should be in the solution. On the back of the conductivity device is a guide for you to use E Tahle II: Electrolytes Lab Results from Conductivity device Compound HNO: 1 Do not write here. Copy this izhle down yowlab notebook HCHI 3 NH NaOH 5 CH(OH). 6 K CO, Cu(NO) 8 Nici: 9 Zn(NO3)2 10 CH OH (othanol) Page 10 > of 19 ZOOM Experimental Procedure for Part : Add approximately 2-3 drops of each pair of reactants in the first column lo a lesi Luhe. Use 0.1 M solutions for all reacliants except when nxitext differently. Record your observations in column Experimental Observations. Be sure to note the formation of a precipitatc, any color change, color of precipitate, evolution of a gas, ate. For a "b" reaction, add 6 drops of the indicated reagent to the precipitate that is left in the well plate to determine if it dissolves. Recxind your observations in your notebook. Table IT: Double Displacement Reactions Reactants Experimental Observations Da nor wire here Coyyy this table down in your lazo narebook la 1b za Bi(NO) + K2SO4) BaSO4(s)+ HCI(12M) ) NiCl + NaCO NiCor+ HCI (12M) 2 38 21 30 de 4a 46 5. Sa Sh CoC +KOH (GM) COH+HCHI2M COOH) THCHIZMI ) + Na PO + ZnS LIST Zn:(PO4)2, +HCK12M) x NASCUNO3)2 + S + H2H4 () Na2COA sald) + HCI(12 M) )2 + NH3 (12M) AgNOX + NaCl AgCl + NH3 (12M) CnChai + NH412M) NH.CI + NaOH 6 7 CNC + NH3 (12) Nh 2 9 10
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


