Question: answer all questions or thumbs down rate MISSED THIS? Read Section 15.2 (Pages 632 - 636) : Watch KCV 15.2. WE 15.1 rate = Consider
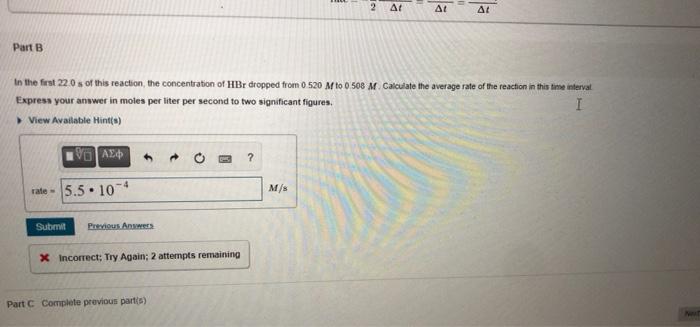
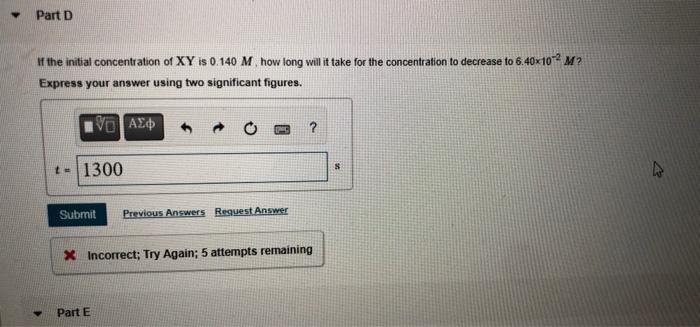
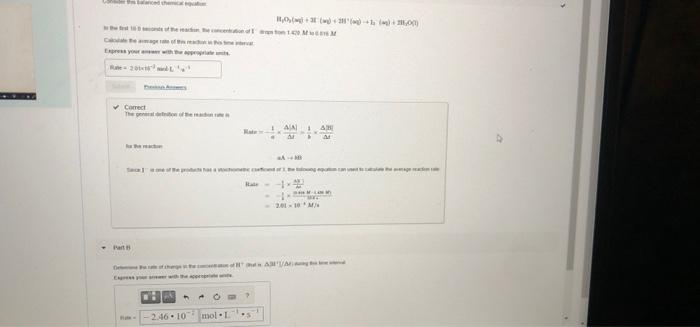
rate MISSED THIS? Read Section 15.2 (Pages 632 - 636) : Watch KCV 15.2. WE 15.1 rate = Consider the following reaction: 2HBr(g) H2(g) + Br2(g) rate = rate = Correct The reac change is reciproca 2. 2 A AL Part B In the first 22.0s of this reaction the concentration of HBr dropped from 0 520 M to 0.508 M Calculate the average rate of the reaction in this time interval Express your answer in moles per liter per second to two significant figures. I View ew Available Hints) V0 AED 3 ? rate - 5.5.10 M/ Submit Previous Answers X Incorrect: Try Again; 2 attempts remaining Part C Complete previous parts) t=4900 MISSED THIS? Read Section 15.4 (Pages 642 - 649. Watch KCV 15.4. WWE 15.4 The decomposition of XY is second order in XY and has a rate constant of 7.14x10- M'. at a certain temperature. 1 Corr Part D If the initial co Express your IV Part D It the initial concentration of XY is 0.140 M how long will it take for the concentration to decrease to 6.40*10-2 M? Express your answer using two significant figures. VO AED ? t-1300 $ 8 Submit Previous Answers Request Answer X Incorrect; Try Again: 5 attempts remaining Part E whom feet. MM Correct AN A 2001- 10 M - AVA 2.46.10. mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


