Question: Answer all the parts to this question plz Write an equation, using the curved-arrow notation, for the acid-base reaction that will take place when each
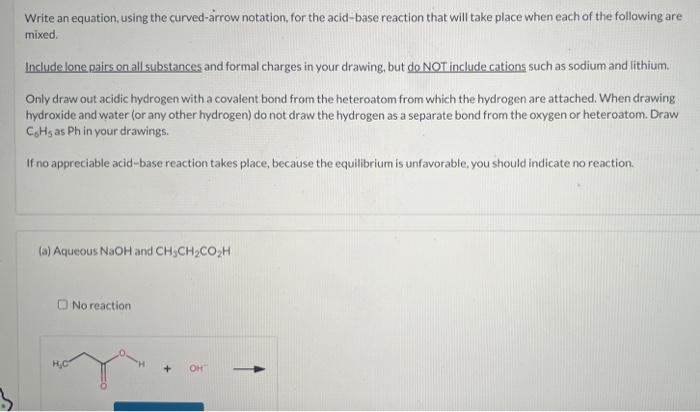
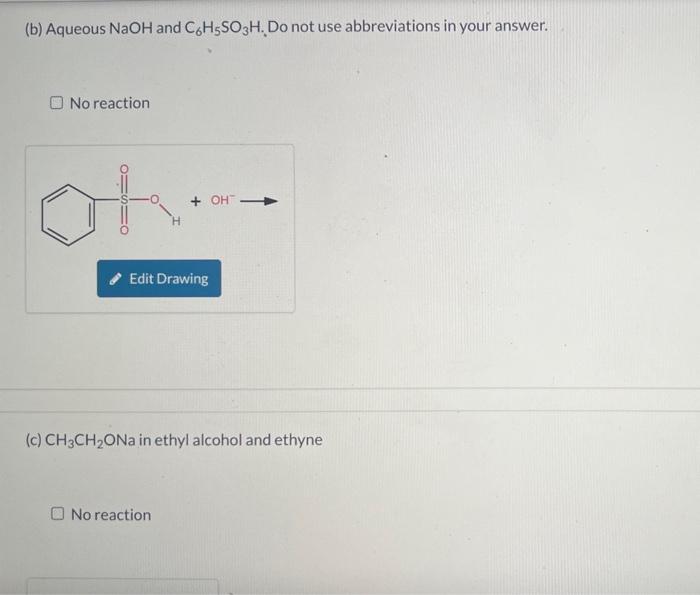
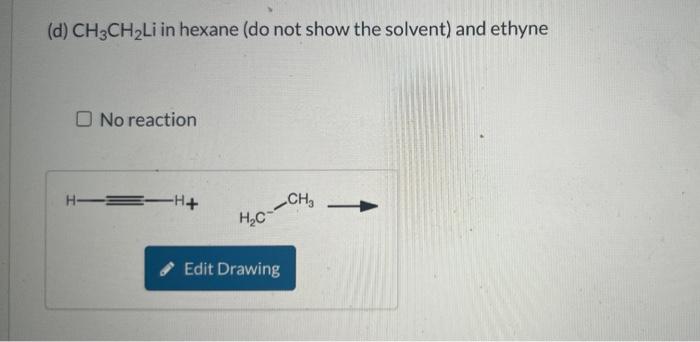
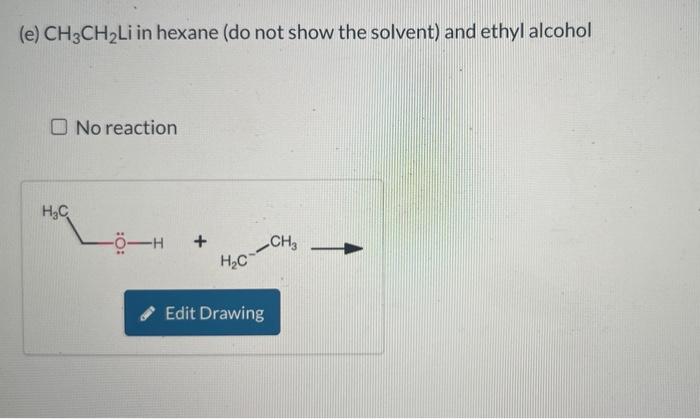
Write an equation, using the curved-arrow notation, for the acid-base reaction that will take place when each of the following are mixed. Include lone pairs on all substances and formal charges in your drawing, but do NOT include cations such as sodium and lithium. Only draw out acidic hydrogen with a covalent bond from the heteroatom from which the hydrogen are attached. When drawing hydroxide and water (or any other hydrogen) do not draw the hydrogen as a separate bond from the oxygen or heteroatom. Draw C6H5 as Ph in your drawings. If no appreciable acid-base reaction takes place, because the equilibrium is unfavorable, you should indicate no reaction. (a) Aqueous NaOH and CH3CH2CO2H No reaction (b) Aqueous NaOH and C6H5SO3H. Do not use abbreviations in your answer. No reaction (c) CH3CH2ONa in ethyl alcohol and ethyne No reaction (d) CH3CH2Li in hexane (do not show the solvent) and ethyne No reaction (e) CH3CH2Li in hexane (do not show the solvent) and ethyl alcohol No reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


