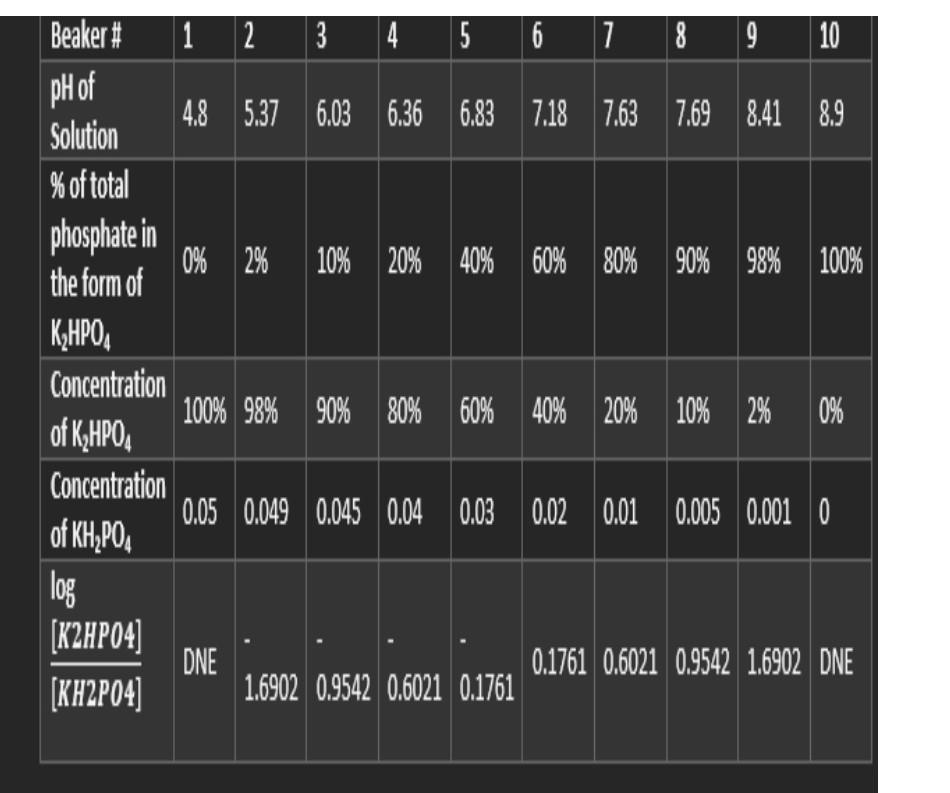
Question: Answer ASAP i will upvote if answer is correct 6. Plot pH vs. % of total phosphate in the form of K,HPO4(Graph 1). [K2HP04] 7.

Answer ASAP i will upvote if answer is correct
6. Plot pH vs. % of total phosphate in the form of K,HPO4(Graph 1). [K2HP04] 7. aPlot log vs. pH (Graph 2). [KH2P04 8. Determine the pKa for the dissociation: H;PO3 HPO2 + H* from each plot . a. pka for H_PO, from Graph 1 = b. pka for HyPO, from Graph 2 = 1 2 3 4 5 6 7 8 9 10 4.8 5.37 6.03 6.36 6.83 7.18 7.63 6.83 7.18 7.637.69 8.41 8.9 0% 2% 10% 20% 40% 60% 80% 40% 60% 80% 90% 98% 100% Beaker # pH of Solution % of total phosphate in the form of K2HPO4 Concentration of KqHPO Concentration of KH,PO. log [K2HPO4] [KH2P04] 100% 98% 90% 80% 60% 40% 20% 60% 40% 20% 10% 2% 0% 0.05 0.049 0.045 0.04 0.03 0.02 0.01 0.03 0.02 0.01 0.005 0.0010 DNE 0.1761 0.6021 0.9542 1.6902 DNE 1.6902 0.9542 0.6021 0.1761
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


