Question: Answer B: 7.5*10^-16 g^2 /cm^4* sec 13 When a sample of a stainless steel (an iron-nickel-chromium alloy) is heated in air, an oxide layer forms
Answer B: 7.5*10^-16 g^2 /cm^4* sec
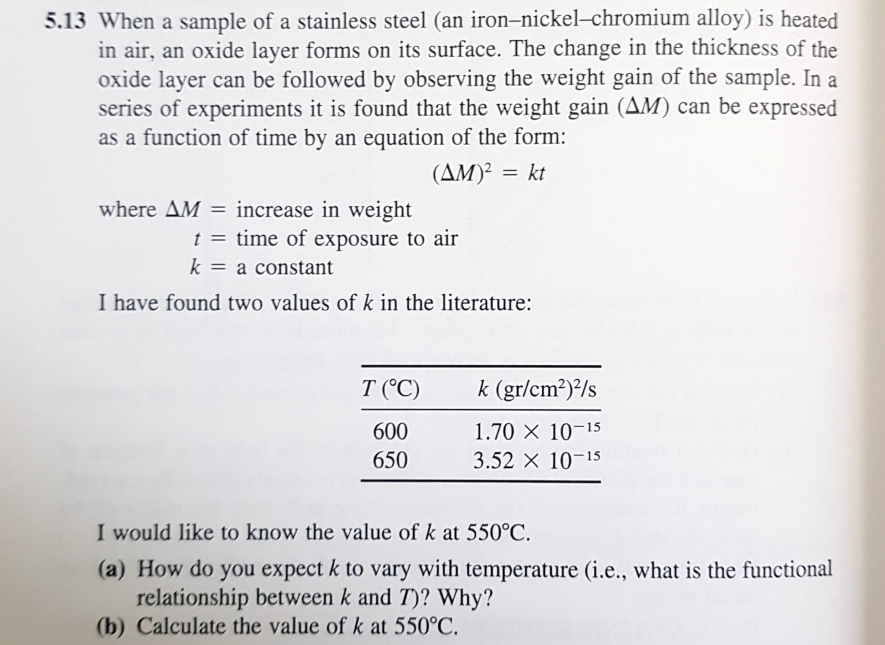
13 When a sample of a stainless steel (an iron-nickel-chromium alloy) is heated in air, an oxide layer forms on its surface. The change in the thickness of the oxide layer can be followed by observing the weight gain of the sample. In a series of experiments it is found that the weight gain (M) can be expressed as a function of time by an equation of the form: (M)2=kt where M= increase in weight t= time of exposure to air k=a constant I have found two values of k in the literature: I would like to know the value of k at 550C. (a) How do you expect k to vary with temperature (i.e., what is the functional relationship between k and T ? Why? (b) Calculate the value of k at 550C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


