Question: answer b, c, and d for part a its not an ideal gas... Argon obeys the following virial equation of state: pV=nRT(1+VnB) with B=21.7cm3mol1 at
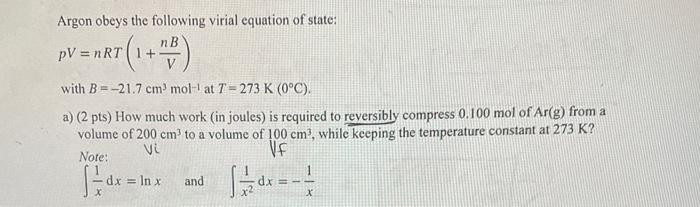

Argon obeys the following virial equation of state: pV=nRT(1+VnB) with B=21.7cm3mol1 at T=273K(0C). a) (2 pts) How much work (in joules) is required to reversibly compress 0.100mol of Ar(g) from a volume of 200cm3 to a volume of 100cm3, while keeping the temperature constant at 273K ? Note: Vi Vf x1dx=lnx and x21dx=x1 b) (1 pt) How much work would have been required if the gas had behaved like a perfect gas? Compare this answer to the answer from part a). c) (1 pt) What is the pressure of the gas once it is compressed? Give your answer in kPa. (Note: 1kPa=1J/dm3=0.001J/cm3.) (Hint: Use the equation of state provided.) d) (1 pt) The virial coefficient B is known to depend on the temperature. Find the value of B at T= 373K(100C), knowing that 0.100mol of Ar(g) at 100C in a volume of 100cm3 has a pressure
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


