Question: answer both pls 3. (20 points) Calculate the standard heat of the following liquid ethanol combustion reaction at (i) 25 C, (ii) 100 C (Hint:
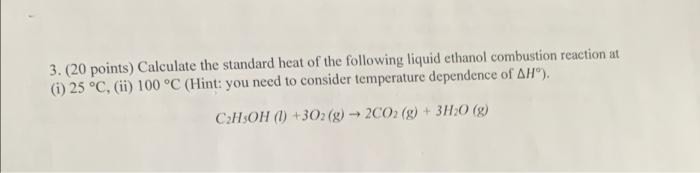

3. (20 points) Calculate the standard heat of the following liquid ethanol combustion reaction at (i) 25 C, (ii) 100 C (Hint: you need to consider temperature dependence of AH). CHOH (l) +302 (9) 2002 (g) + 3H20 (9) 4. (30 points) 50.0 g of ice at -10 C is added to 900 g of H20 (1) at 90 C. The process is carried out in an isolated container under constant pressure of 1 bar. Calculate the final temperature of the system and AS for the overall process. Is the process spontaneous or not? The heat capacities and enthalpies are given in the following: Cm(H2000) = 75.3 J K-moll and CP(H2O(s)) = 37.7 J K 'mol'', AH fusion 6 kJ molat 0 C. Molecular weight of water is 18 g mol' (Hint: assume constant heat capacities in this problem)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


