Question: ANSWER BOTH [References] 13 1 pt TUTOR Determining Molecular Formulas from Percent Composition 1 pt An organic acid is composed of carbon (58.80%), hydrogen (9.89%),
![ANSWER BOTH [References] 13 1 pt TUTOR Determining Molecular Formulas from Percent](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8e0f5da029_54966f8e0f5387ca.jpg)
 ANSWER BOTH
ANSWER BOTH
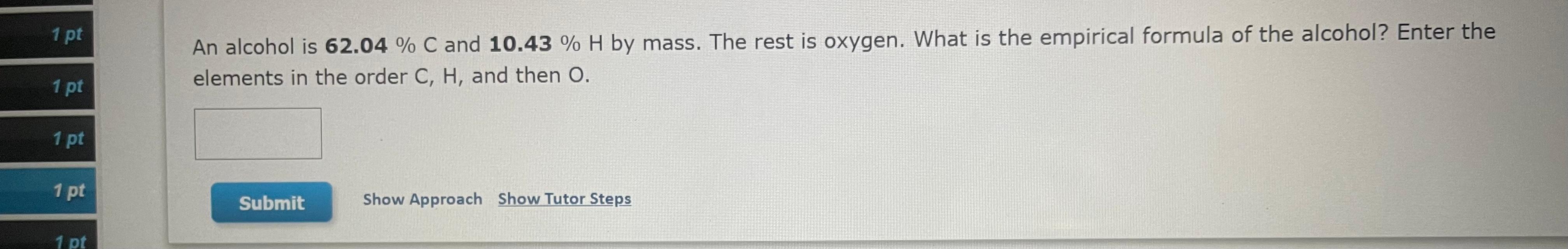
[References] 13 1 pt TUTOR Determining Molecular Formulas from Percent Composition 1 pt An organic acid is composed of carbon (58.80%), hydrogen (9.89%), and oxygen (31.33%). Its molecular weight is 102.13 amu. Determine the molecular formula of the compound. 1 pt 1 pt 1 pt Submit Show Approach Show Tutor Steps 1 pt 1 pt An alcohol is 62.04 % C and 10.43 % H by mass. The rest is oxygen. What is the empirical formula of the alcohol? Enter the elements in the order C, H, and then 0. 1 pt 1 pt 1 pt Submit Show Approach Show Tutor Steps 1 pt
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


