Question: answer Chrome File Edit View History Bookmarks Profiles Tab Window 0 0 7 9 8 0 Sun Dec 1 3:35 PM Dasht X B At
answer
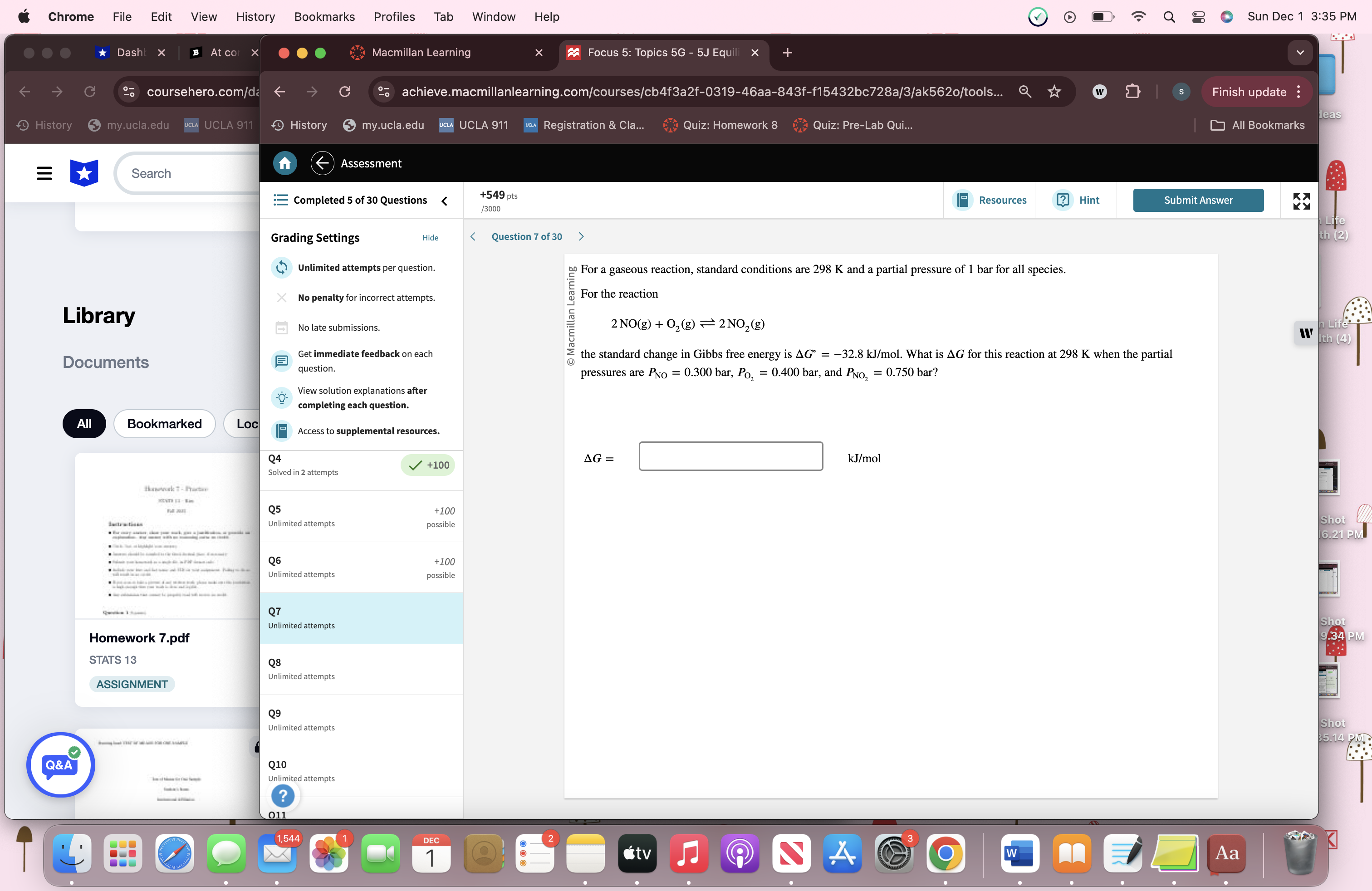
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


